Abstract
BACKGROUND:
While treatment guidelines exist for melanoma in situ and invasive melanoma, guidelines for other melanocytic skin lesions do not exist.
OBJECTIVE:
To examine pathologists’ treatment suggestions for a broad spectrum of melanocytic skin lesions and in comparison with existing guidelines.
METHODS:
Pathologists (N=187) completed a survey and then provided diagnoses and treatment suggestions for 240 melanocytic skin lesions. Physician characteristics associated with treatment suggestions were evaluated using multivariable modeling.
RESULTS:
Treatment suggestions were concordant with National Comprehensive Cancer Network (NCCN) guidelines for the majority of cases interpreted as melanoma in situ (73%) and invasive melanoma (86%). Greater variability of treatment suggestions was seen for other lesion types without existing treatment guidelines. Characteristics associated with provision of treatment suggestions discordant with NCCN guidelines were: low caseloads (invasive melanoma), lack of fellowship training or board certification (melanoma in situ), and >10 years of experience (invasive melanoma and melanoma in situ).
LIMITATIONS:
Pathologists could not perform immunohistochemical staining or other diagnostic tests; only one glass side provided per biopsy case.
CONCLUSIONS:
Pathologists’ treatment suggestions vary significantly for melanocytic lesions, with lower variability for lesion types with national guidelines. Results suggest the need for standardization of treatment guidelines for all melanocytic lesion types.
Introduction:
While clinicans ultimately determine patient treatment for melanocytic skin lesions (MSLs), factoring histopathology, dermoscopy, clinical suspicion, and clinical history, the pathologists may typically be the only provider examining a skin biopsy specimen and therefore can also serve as an important resource on guiding optimal treatment options. Our study follows-up on a national survey which found that a majority of skin pathologists reported providing treatment suggestions along with their biopsy interpretations of MSL on some of their reports.1 Although national guidelines exist for the management of melanoma, there is less agreement for the treatment of other types of MSL (e.g., atypical/dysplastic nevi)2–5.
As a result of striking variability among pathologists in the diagnosis of melanocytic lesions.6–10, we hypothesized that there would also be extensive variation in treatment recommendations given for MSLs, even when controlling for the diagnosis provided by the pathologists. Since many patients undergoing skin biopsies have dysplasic nevi or borderline diagnoses where no treatment guidelines exist, or the patients are evaluated and treated by nonspecialized clinicians such as primary care physicians, we suspect that variation in pathologists’ treatment suggestions could lead to extensive variation in clinical care.11,12 About one in five skin biopsies obtained in the U.S. are of melanocytic lesions, highlighting the commonality of these lesions in clinical practice8 and thus the significance of this topic.
In this report, we examine the variability in treatment suggestions regarding reexcision and margin width for a given diagnosis, specifically looking at pathologists’ treatment suggestions for melanoma in situ and invasive melanoma cases and concordance with national guidelines for treatment.13 In addition, we examine concordance of pathologists’ treatment suggestions for other types of MSLs (e.g., dysplastic nevi, Spitz lesions) and concordance of treatment suggestions by pathologists and the MPATH-Dx treatment suggestions14. This study, which presents data on pathologists’ treatment recommendations during the actual interpretation of skin biopsies, follows up on our prior survey study of pathologists’ views regarding appropriate treatments for differing types of MSLs.3
Methods:
Data derive from the M-Path study, a national project examining the accuracy and reproducibility of pathologists’ interpretations of MSLs.9 IRB approval was acquired and informed consent was obtained from each participant. M-Path methods have been previously reported in detail.9,14–16
Study Pathologists Identification, Recruitment, and Baseline Characteristics:
Pathologists from 10 U.S. states were identified using publicly available information from professional organizations and updated through internet searches and telephone calls. Pathologists were then invited by email, with postal and telephone follow-up. Pathologists were informed that they would receive a confidential, individualized report of their interpretive results alongside those of their peers and an expert panel, and free CME credit (up to 20 hours category 1).Eligibility criteria included self-reported interpretation of MSL over the past year, and an expectation to continue interpreting MSLs over the two subsequent years. The study excluded residents and fellows. Pathologists completed a baseline survey regarding demographics.
Case Selection and Composition
The skin biopsy cases were of cutaneous melanocytic lesions from shave, punch, and excisional specimens. Cases were initially selected using stratification on patient age (20–49 years, 50–64 years, ≥65 years) and medical chart documentation of the original diagnosis in order to achieve the desired target distribution of cases across MPATH-Dx diagnostic categories and an even distribution of cases across these three age groups within each diagnostic category. One single slide for each case was used. Three expert dermatopathologists (RB, DE, MP) independently reviewed the new H&E stained glass slides for each case, followed by a consensus review using a modified Delphi Approach17. The final 240 cases were then selected with the desired distribution across MPATH-Dx categories for this consensus diagnosis and with an even distribution across age categories. The final 240 cases had intentionally higher proportions in MPATH-Dx Classes II-V than typically found in clinical practice with distribution as follows: 10.4% (n=25) in class I, 15% (n=36) in class II, 25% (n=60) in class III, 22.9%(n=55) in class IV, and 25.4% (n=61) in class V. The final 240 cases were assembled into five sets of 48 cases.
Case Interpretation by Pathologists:
After completing the survey, pathologists were randomized to receive one of five sets of 48 MSL glass slides developed for the M-Path study. A permutated block randomization with stratification on pathologists’ clinical expertise was used. Expertise was dichotomized based on possession of one or more of the following self-reported characteristics on the baseline survey: fellowship trained or board certified in dermatopathology; considered by colleagues to be an expert in melanocytic lesions; or 10% or more of usual caseload included cutaneous melanocytic lesions. Slides were presented in a random order to each participant.
Using the online MPATH-Dx form, pathologists reported their diagnoses and treatment suggestions for each case.14 Participants were provided with the patient’s age, sex, biopsy type, and anatomic location of the biopsy site on each online histology form for a case. Standardized diagnostic definitions or educational materials were not provided for participants. Participants were also asked to assume that the single glass slide was representative of the entire lesion and that the margin was positive.
Participants could provide their treatment recommendation for a case at any time during their review of the slide. The pathologists were asked “Although final treatment decisions may rest with the clinician and the patient, what clinical course would you suggest for consideration?” and were given four possible options: a. No further treatment; b. Excision <0.5cm; c. Excision 0.5cm to <1cm; d. Excision ≥ 1 cm. Pathologists were told to assume the glass slide was representative of the lesion as a whole, and that the lesion extended all the way to the edge of the sample (i.e., if there were any residual lesion beyond the edge of the specimen it would not be different from what was already on the slide). These assumptions were stipulated so as to obligate participants to consider the four MPATH-Dx treatment options for every lesion, because otherwise they would not recommend re-excision for completely excised lesions, except for melanomas.
Statistical Analysis:
A classification scheme for melanocytic lesions, the Melanoma Pathology Assessment Tool and Hierarchy for Diagnosis (MPATH-Dx) tool14, was used in this study.10,14 The MPATH-Dx tool moves beyond National Comprehensive Cancer Network (NCCN) guidelines by providing treatment suggestions for the full spectrum of MSLs. The MPATH-Dx classification tool categorizes interpretations into five overarching diagnostic classes,14 with example diagnoses and corresponding treatment suggestions as follows: I) nevus/mild dysplastic nevi (no further treatment required); II) moderate dysplastic nevi (narrow but complete re-excision <0.5 cm); III) severe dysplastic nevi/melanoma in situ (repeat excision with ≥0.5 cm but < 1cm margins); IV) T1a invasive melanoma (wide excision ≥ 1cm) and V) >= T1b invasive melanoma (wide excision ≥ 1cm, and consideration of sentinel lymph node staging or adjuvant therapy).
A wide spectrum of diagnostic terms are used in clinical practice for melanocytic lesions9. The MPATH-Dx system classifies some diagnostic terms on the basis of the treatment suggested by the pathologists ( e.g., MELUMP, Melanocytic Lesion of Uncertain Malignant Potential might be classified as an MPATH-Dx class II, III,IV or V based on the suggested treatment indicated by the interpreting pathologist). As this is a study of suggested treatment for more clearly specified diagnostic terms, 477 interpretations receiving variable diagnostic designations such as the latter were excluded.
In the analyses, cases given a Class III diagnosis were divided into two separate sub-groups; to wit, melanoma in situ and all other Class III cases (e.g. severely dysplastic nevus, atypical Spitz tumor, atypical nevus, lentiginous melanocytic proliferation with severe atypia), to allow comparison of treatment suggestions for cases interpreted as melanoma in situ per NCCN guidelines.18 Invasive melanoma cases categorized into MPATH-Dx class IV and V were combined because we limited our study to recommendations on local excision procedures and did not consider additional details such as sentinel lymph node staging or adjuvant therapy.
We placed greater emphasis on treatment suggestions for melanoma in situ and invasive melanoma because national guidelines provide precedent for these diagnoses. Treatment suggestions for melanoma in situ were parsed as a 3-category ordinal variable: suggested re-excision less than 0.5cm, suggested re-excision between 0.5cm and <1cm, and suggested re-excision >=1 cm. Treatment suggestions for invasive melanoma were dichotomized as suggested re-excision less than 1cm versus suggested re-excision 1cm or greater. Multivariable modeling to examine associations between physician characteristics and treatment suggestions employed generalized estimating equations accounting for repeated measures. SAS version 9.4 (SAS Institute Inc, Cary, NC) was used to conduct all analyses.
Results:
Of the 207 pathologists who completed the baseline survey, 187 (90.3%) completed the diagnostic interpretations. The average pathologist was 51 years old (range: 33–79). Most pathologists were not affiliated with a medical center (72%), did not hold fellowship or board-certification in dermatopathology (60%), had greater than ten years of experience interpreting MSL (60%), and reported high confidence in their own abilities to assess MSL (86%).The 187 pathologists, blinded to each others’ interpretations, provided a total of 8,499 interpretations used in this analysis.
The majority of treatment suggestions by these pathologists aligned with the original published MPATH-Dx suggested management for each Class.9 Pathologists had highest agreement between treatment suggestions for cases they diagnosed as Class I (interpreted as benign cases with 81.6% suggesting no further treatment) and Class IV/V (interpreted as invasive melanoma with 86.1% suggesting margins of ≥1cm). While cases diagnosed as Class II and Class III lesions exhibited greater variability in the participants’ corresponding treatment suggestions, the majority of the participants’ treatment suggestions were aligned with the MPATH-Dx class suggested treatment: 65.8% (Class II); 50.9% (Class III, Other); 72.8% (Class III, Melanoma in situ).
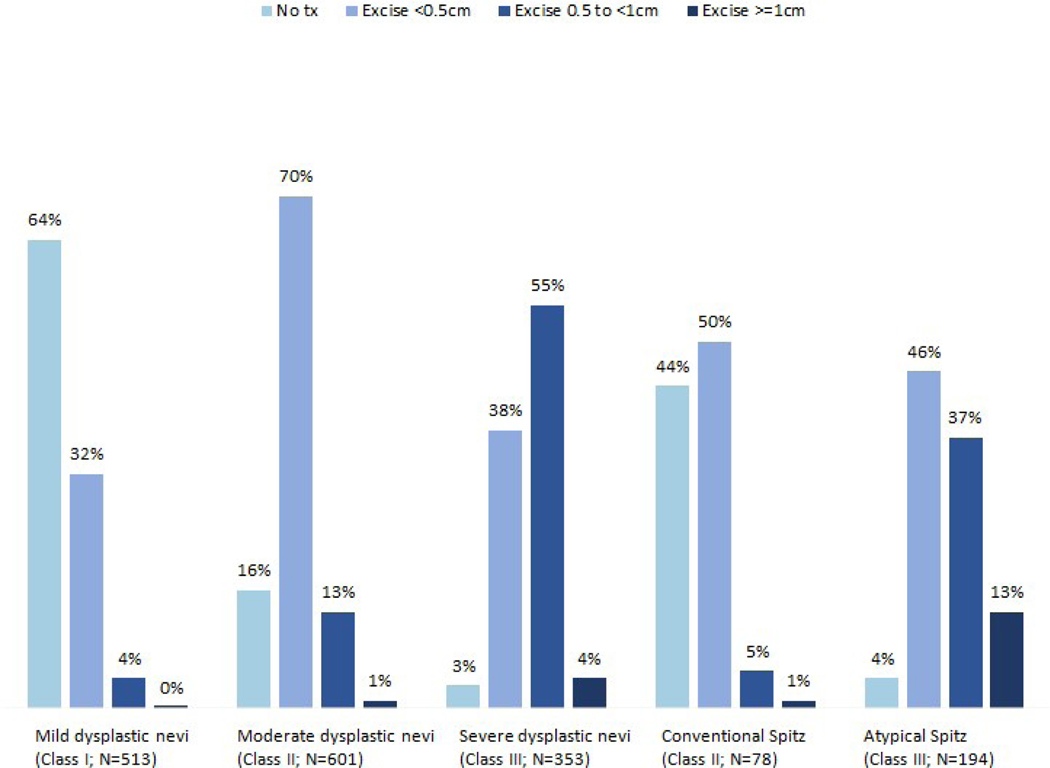
The pathologists’ treatment suggestions for diagnoses of dysplastic nevi (mild, moderate or severe) and Spitz nevus (conventional or atypical) are illustrated in Figure 1. The majority of pathologists suggested no treatment for mildly dysplastic nevi (64%), <0.5cm excision for moderately dysplastic nevi (70%), and 0.5 to <1cm excision for severely dysplastic nevi (55%). There was less agreement for treatment suggestions for both conventional and atypical Spitz tumors.
Figure 1:
Pathologist treatment suggestions for cases diagnosed as dysplastic nevi and Spitz tumors. N’s represent total interpretations.
Figure 2 provides images and participants’ treatment suggestions for two melanoma in situ study cases (reference diagnosis of melanoma in situ defined by a panel of three highly experienced dermatopathologists; MIS diagnosis agreed upon by 17 out of 18 participants). For Case A, 29% of participating pathologists suggested a lower treatment (<0.5cm) and 18% suggested a higher treatment (≥ 1cm margin) than NCCN guidelines, showing relatively large variability in treatment suggestions. In contrast, Case B had more consistent treatment suggestions. For Case A, the pathological changes were subtle and required examination at higher magnification to notice pagetoid scatter and the poor circumscription. Case B, however, was a more circumscribed, larger lesion with easily identified nested growth at low magnification.
Figure 2: Two Examples of Melanoma In Situ Glass Slide Biopsies evoking High and Low Variability in Treatment Suggestions by the Study Participants.
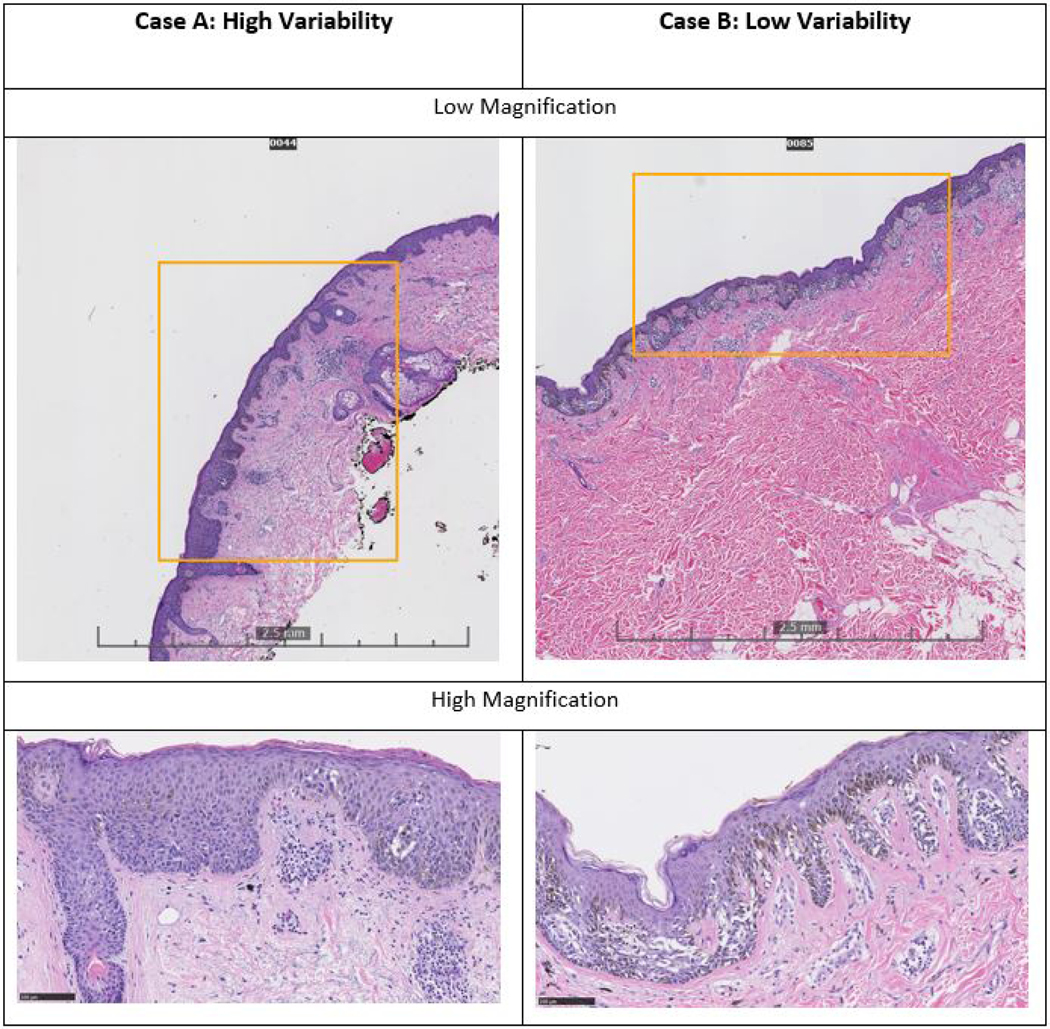
Both cases were diagnosed as melanoma in situ by consensus panel and 17 out of 18 pathologists. For Case A, 29% of participating pathologists suggested a lower treatment (<0.5cm) and 18% suggested a higher treatment (≥ 1cm margin) than NCCN guidelines, showing relatively large variability in treatment suggestions. Case A is relatively small (~4mm) and does not have an obviously atypical melanocytic proliferation at low magnification. Higher magnification allows the identification of a poorly circumscribed intraepidermal melanocytic proliferation. In contrast, Case B had a smaller proportion of pathologists (12%) rendering a treatment suggestion lower than NCCN guidelines, and no pathologists suggested a higher treatment than NCCN guidelines. Case B is a larger lesion (~8mm) that more readily is identifiable as an atypical lentiginous and nested proliferation with pagetoid scatter.
Table 1 displays the variation in treatment suggestions for melanoma in situ and invasive melanoma by pathologist characteristics. All characteristics in Table 1 are significantly associated (p<0.01) with variation in treatment suggestions for invasive melanoma. Three characteristics (board-certification and/or fellowship-training in dermatopathology, fewer years of experience, and greater caseload of MSLs) associate with higher agreement with NCCN guidelines for melanoma in situ.
Table 1:
Distribution of Treatment Suggestions for Melanoma In Situ and Invasive Melanoma Case Diagnoses by Pathologist Characteristics
| Melanoma in situ treatment suggestion | IM treatment suggestiona | |||||
|---|---|---|---|---|---|---|
| excision | excision | Excision | excision | Excision | ||
| < 0.5cm | 0.5-<1cmb | ≥ 1cm | < 1cm | ≥ 1cmb | ||
| No. case readings | 111 | 665 | 137 | 438 | 2711 | |
|
| ||||||
| Affiliation with academic medical center | ||||||
| No | 2833 | 80 (13%) | 449 (72%) | 96 (15%) | 339 (15%) | 1869 (85%) |
| Yes | 1229 | 31 (11 %) | 216 (75%) | 41 (14%) | 99 (11 %) | 842 (89%) |
| Fellowship or board certified in dermatopathology | ||||||
| No | 2308 | 89 (17%) | 330 (62%) | 114 (21%) | 328 (18%) | 1447 (82%) |
| Yes | 1754 | 22 (6%) | 335 (88%) | 23 (6%) | 110 (8%) | 1264 (92%) |
| Years interpreting melanocytic skin lesions | ||||||
| < 10 years | 1583 | 19 (6%) | 273 (80%) | 50 (15%) | 56 (5%) | 1185 (95%) |
| 10 years + | 2479 | 92 (16%) | 392 (69%) | 87 (15%) | 382 (20%) | 1526 (80%) |
| Percent of caseload represented by melanocytic skin lesions | ||||||
| < 10 percent | 1581 | 62 (17%) | 219 (61%) | 76 (21 %) | 250 (20%) | 974 (80%) |
| ≥ 10 percent | 2481 | 49 (9%) | 446 (80%) | 61 (11 %) | 188 (10%) | 1737 (90%) |
IM=invasive melanoma
NCCN guidelines for surgical margins for primary melanoma (exceptions to these guidelines exist for both melanoma in situ and invasive melanoma).
Multivariable analysis of the pathologist characteristics data assessed the concordance/discordance parameter by reference to NCCN guidelines (Table 2). Pathologists with a greater number of years interpreting MSL (10+ years) are significantly less likely to provide treatment suggestions concordant with NCCN guidelines for invasive melanoma, while pathologists with a high caseload are significantly more likely to provide treatment suggestions concordant with those guidelines. Pathologists with fellowship-training or board-certification in dermatopathology were significantly more likely to provide treatment suggestions consistent with the guidelines for melanoma in situ after adjusting for the other parameters. More years in clinical practice correlate with treatment suggestions less than NCCN guidelines for melanoma in situ, and affiliation with an academic medical center correlates with suggestions greater than those guidelines.
Table 2:
Multivariable Analysis Examining Pathologist Characteristics Associated with Treatment Suggestions as Referenced to National Comprehensive Cancer Network (NCCN)
| Likelihood of Suggesting Treatment Concordant with NCCN Guidelines for Invasive Melanomaa | Likelihood of Suggesting Lesser Treatment than NCCN Guidelines for Melanoma In Situa | Likelihood of Suggesting Greater Treatment than NCCN Guidelines for Melanoma In Situa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio | Lower Confidence | Upper Confidence | Odds Ratio | Lower Confidence | Upper Confidence | Odds Ratio | Lower Confidence | Upper Confidence |
| Affiliated with academic medical center | 1.27 | 0.95 | 1.7 | 1.21 | 0.76 | 1.93 | 1.61 * | 1.06 | 2.44 |
| Fellowship or board certified in dermatopathology | 1.24 | 0.97 | 1.58 | 0.33 * | 0.18 | 0.63 | 0.17 * | 0.09 | 0.32 |
| Years interpreting melanocytic skin lesions (10+ years) | 0.21 * | 0.16 | 0.29 | 2.35 * | 1.30 | 4.24 | 0.75 | 0.48 | 1.16 |
| Percent of caseload represented by melanocytic skin leions (10%+ of caseload) | 1.70 * | 1.39 | 2.08 | 0.76 | 0.48 | 1.20 | 0.84 | 0.56 | 1.27 |
Odds ratios are adjusted odds ratios (adjusted for the other variables in the multivariable model).
Statistically significant at p ≤ 0.05
Pathologists’ characteristics associated with treatment recommendations for cases they interpreted as moderate and severe atypia, or as conventional and atypical Spitz are shown in Tables 3 and 4, respectively. Pathologists working in academic medical centers, those with fellowship training or board certification in dermatopathology, and those with ≥10 percent of caseload in melanocytic skin lesions tended to be more likely to suggest no margins for moderate atypia. Pathologists who were fellowship trained or board certified in dermatopathology, and those who had less than 10 years interpreting melanocytic skin lesions were more likely to suggest lower margins (≤.5) for severe dysplasia. Pathologists affiliated with an academic center were more likely to suggest a smaller margin for atypical Spitz lesions.
Table 3:
Suggested Treatment Recommendations for Cases Interpreted as Moderate or Severe Dysplasia by Pathologists’ Characteristics.
| Moderate dysplasia | Severe dysplasia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| No. case readings | None | ≤ .5 | .5–1 | ≥ 1 | P-value | None | ≤ .5 | .5–1 | ≥ 1 | P-value | |
| 99 | 420 | 79 | 3 | 10 | 134 | 193 | 16 | ||||
|
| |||||||||||
| Affiliation with academic medical center | |||||||||||
| No | 6432 | 59 (14%) | 316 (72%) | 60 (14%) | 1 (0%) | <0.01 | 6 (2%) | 99 (40%) | 130 (53%) | 12 (5%) | 0.51 |
| Yes | 2544 | 40 (24%) | 104 (63%) | 19 (12%) | 2 (1 %) | 4 (4%) | 35 (33%) | 63 (59%) | 4 (4%) | ||
| Fellowship or board certified in dermatopathology | |||||||||||
| No | 5424 | 35 (10%) | 235 (69%) | 70 (20%) | 3 (1 %) | <0.001 | 6 (3%) | 67 (35%) | 107 (55%) | 14 (7%) | 0.03 |
| Yes | 3552 | 64 (25%) | 185 (72%) | 9 (3%) | 0 (0%) | 4 (3%) | 67 (42%) | 86 (54%) | 2 (1 %) | ||
| Years interpreting melanocytic skin lesions | |||||||||||
| < 10 years | 3552 | 39 (16%) | 182 (73%) | 27 (11%) | 2 (1 %) | 0.34 | 2 (1 %) | 44 (28%) | 100 (65%) | 9 (6%) | <0.01 |
| 10 years + | 5424 | 60 (17%) | 238 (68%) | 52 (15%) | 1 (0%) | 8 (4%) | 90 (45%) | 93 (47%) | 7 (4%) | ||
| Percent of caseload is melanocytic skin lesions | |||||||||||
| < 10 percent | 3792 | 27 (11%) | 173 (72%) | 40 (17%) | 0 (0%) | <0.01 | 4 (3%) | 52 (36%) | 83 (57%) | 6 (4%) | 0.87 |
| ≥ 10 percent | 5184 | 72 (20%) | 247 (68%) | 39 (11%) | 3 (1 %) | 6 (3%) | 82 (39%) | 110 (53%) | 10 (5%) | ||
Table 4.
Suggested Treatment Recommendations for Cases Interpreted as Conventional Spitz or Atypical Spitz by Pathologists’ Characteristics.
| Conventional Spitz | Atypical Spitz | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| No. case readings | None | ≤ .5 | .5–1 | ≥ 1 | P-value | None | ≤ .5 | .5–1 | ≥ 1 | P-value | |
| 34 | 39 | 4 | 1 | 8 | 90 | 71 | 25 | ||||
|
| |||||||||||
| Affiliation with academic medical center | |||||||||||
| No | 6432 | 23 (43%) | 28 (53%) | 2 (4%) | 0 (0%) | 0.35 | 3 (2%) | 65 (45%) | 59 (40%) | 19 (13%) | 0.04 |
| Yes | 2544 | 11 (44%) | 11 (44%) | 2 (8%) | 1 (4%) | 5 (10%) | 25 (52%) | 12 (25%) | 6 (13%) | ||
| Fellowship or board certified in dermatopathology | |||||||||||
| No | 5424 | 19 (42%) | 22 (49%) | 3 (7%) | 1 (2%) | 0.89 | 4 (4%) | 47 (46%) | 38 (37%) | 14 (14%) | 0.98 |
| Yes | 3552 | 15 (45%) | 17 (52%) | 1 (3%) | 0 (0%) | 4 (4%) | 43 (47%) | 33 (36%) | 11 (12%) | ||
| Years interpreting melanocytic skin lesions | |||||||||||
| < 10 Years | 3552 | 8 (31%) | 17 (65%) | 1 (4%) | 0 (0%) | 0.24 | 2 (2%) | 41 (48%) | 32 (37%) | 11 (13%) | 0.78 |
| 10 years + | 5424 | 26 (50%) | 22 (42%) | 3 (6%) | 1 (2%) | 6 (6%) | 49 (45%) | 39 (36%) | 14 (13%) | ||
| Percent of caseload is melanocytic skin lesions | |||||||||||
| < 10 percent | 3792 | 18 (50%) | 15 (42%) | 2 (6%) | 1 (3%) | 0.34 | 2 (3%) | 32 (45%) | 27 (38%) | 10 (14%) | 0.9 |
| ≥ 10 percent | 5184 | 16 (38%) | 24 (57%) | 2 (5%) | 0 (0%) | 6 (5%) | 58 (47%) | 44 (36%) | 15 (12%) | ||
Discussion:
This study highlights extensive variability in treatment suggestions provided by practicing U.S. pathologists as they diagnose MSLs, even while controlling for their diagnostic interpretation of the case. The greatest variability occurs with lesions that have no standardized national treatment guidelines: MPATH-Dx Class II (e.g., moderately dysplastic nevi) and Class III diagnoses other than melanoma in situ (such as severely dysplastic nevi, atypical Spitz nevus/tumor)4,11,14. Adverse consequences resulting from treatment variability of the Class II and III cases are likely to be rare events. We observed less variation in treatment suggestions for diagnoses of melanoma in situ and invasive melanoma, which have NCCN treatment guidelines, and where adherence to treatment recommendations is more consequential.13 The higher variability for treatment suggestions accompanying diagnoses of severely dysplastic nevi compared to mildly and moderately dysplastic nevi may reflect disagreement within the field regarding their biological significance and appropriate treatment. The lack of standardized treatment guidelines for many of these melanocytic lesions has similar implications.
Our analysis indicates that 10 or more years of experience interpreting MSLs is positively associated with providing treatment suggestions that are discordant with national guidelines, suggesting that more recent training may lead to better familiarity with the current guidelines.19 Notably, national guidelines for treatment of invasive melanomas have changed over time. However, since 200918 these recommendations have remained largely unchanged, although there has been discussion regarding margin widths for lentigo maligna melanoma in situ, especially those on the face.12
Our analysis also highlights increased concordance with national guidelines for those pathologists who interpret a higher volume of melanocytic lesion cases. This relationship may relate to the increased motivation of pathologists who spend more time interpreting these lesions to be concordant with the national guidelines.
While a previous M-Path study reported variation in pathologists’ treatment suggestions based on self-reported survey data,1 this study provides new data on variability in the treatment suggestions that accompany actual interpretations of these lesions. Our results further expand the conclusions of the previous study, revealing evidence for substantial variability in treatment suggestions across all lesions, and in particular for those lesions not having national guidelines for their treatment.
Strengths and Limitations:
The limitations to our investigations are the test setting for interpretations; there was only one glass slide for each skin biopsy case (although pathologists were asked to assume it was representative); and pathologists were unable to perform immunohistochemical staining or other diagnostic tests. In addition, pathologists were not provided detailed clinical history for the cases, and they were not able to procure a second opinion if desired. Pathologists were also given only four different options for treatment suggestions, which may have limited the ability to fully communicate their suggestions. Furthermore, we are currently refining the MPATH-Dx schema, in light of new research evidence on Class II and III categories, and we are aware there is disagreement on some of the MPATH-Dx classifications and their respective treatment recommendations2–5.
Strengths of our study include the broad spectrum and high number of cases and the large number of participating pathologists from across the U.S. While other studies have found variation in diagnostic interpretations of melanocytic lesions between pathologists6–9,20–31, our study is unique in that it quantifies variation in treatment suggestions. Our study also identified pathologist characteristics associated with providing treatment suggestions that are discordant with national guidelines.
Clinical and Policy Implications:
This study suggests that there may be a potential benefit from implementation of a standardized taxonomy, such as MPATH-Dx, which aims to integrate pathologists’ diagnostic interpretations with corresponding treatment suggestions. It is encouraging that the majority of treatment recommendations made by these pathologists aligned with the original published MPATH-Dx suggested management for each Class.9 We encourage further refinement and updates on the MPATH-Dx schema, as we are currently doing in light of new research findings. Our hope is that this tool could support primary care clinicians who might be confused by pathology reports on MSL and reduce the observed variability in pathologists’ evaluation of MSL and assist in aligning treatment with national guidelines.
Furthermore, our results suggest that clinicans should bear in mind the significant variability present in treatment recommendations, especially for diagnoses with no standardized national guidelines. Clinical practice guidelines are needed, and are now being suggested through collaborations in dermatology and dermatopathology.9
Finally, this study suggests the need for a more robust continuing education system to inform pathologists about developments in the field. The positive association between fewer years of experience interpreting melanocytic lesions and concordance with national guidelines indicates that pathologists who recently trained are more likely to be aware of current treatment guidelines. Further developments in continuing medical education could improve standardization of patient care and conformity with evidence-based standards where these exist.
Conclusion:
Our study identifies substantial variability in treatment suggestions provided by pathologists when interpreting MSLs. The variability is less for lesions for which there are national treatment guidelines and when interpreted by pathologists with fellowship training or board certification in dermatopathology, suggesting the potential for educational opportunities and national guidelines to improve the concordance of treatment.
Capsule Summary—
National treatment guidelines exist for managing in situ and invasive melanoma, but not for other melanocytic lesions.
Pathologists’ treatment suggestions were found to vary significantly for melanocytic lesions, with lower variability for lesion types with national guidelines and when interpreted by pathologists with fellowship training or board certification in dermatopathology.
Acknowledgement:
We thank the study participants for their commitment to improving clinical care in dermatopathology and the JAAD reviewers for their helpful suggestions.
Funding: Supported by the National Cancer Institute (R01 CA151306, R01 CA 200690, R01 CA201376) and the University of Washington Medical Student Research Training Program (MSRTP). The funding agency had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: Parts of the data and analyses included in this study were presented at the Western Medical Research Conference (2018) and the American Society of Dermatopathology Annual Meeting (2018).
Conflict of Interests: The authors do not have any financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations:
- 1.Lee KC, Peacock S, Weinstock MA, et al. Variation among pathologists’ treatment suggestions for melanocytic lesions: A survey of pathologists. J Am Acad Dermatol. 2017;76(1):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hocker TL, Alikhan A, Comfere NI, Peters MS. Favorable long-term outcomes in patients with histologically dysplastic nevi that approach a specimen border. J Am Acad Dermatol. 2013;68(4):545–551. [DOI] [PubMed] [Google Scholar]
- 3.Goodson AG, Florell SR, Boucher KM, Grossman D. Low rates of clinical recurrence after biopsy of benign to moderately dysplastic melanocytic nevi. J Am Acad Dermatol. 2010;62(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming NH, Egbert BM, Kim J, Swetter SM. Reexamining the Threshold for Reexcision of Histologically Transected Dysplastic Nevi. JAMA Dermatol. 2016;152(12):1327–1334. [DOI] [PubMed] [Google Scholar]
- 5.Engeln K, Peters K, Ho J, et al. Dysplastic nevi with severe atypia: Long-term outcomes in patients with and without re-excision. J Am Acad Dermatol. 2017;76(2):244–249. [DOI] [PubMed] [Google Scholar]
- 6.Duncan LM, Berwick M, Bruijn JA, Byers HR, Mihm MC, Barnhill RL. Histopathologic recognition and grading of dysplastic melanocytic nevi: an interobserver agreement study. J Invest Dermatol. 1993;100(3):318S–321S. [DOI] [PubMed] [Google Scholar]
- 7.Duray PH, DerSimonian R, Barnhill R, et al. An analysis of interobserver recognition of the histopathologic features of dysplastic nevi from a mixed group of nevomelanocytic lesions. J Am Acad Dermatol. 1992;27(5 Pt 1):741–749. [DOI] [PubMed] [Google Scholar]
- 8.Corona R, Mele A, Amini M, et al. Interobserver variability on the histopathologic diagnosis of cutaneous melanoma and other pigmented skin lesions. J Clin Oncol. 1996;14(4):1218–1223. [DOI] [PubMed] [Google Scholar]
- 9.Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lott JP, Elmore JG, Zhao GA, et al. Evaluation of the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis (MPATH-Dx) classification scheme for diagnosis of cutaneous melanocytic neoplasms: Results from the International Melanoma Pathology Study Group. Journal of the American Academy of Dermatology. 2016;75(2):356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CC, Swetter SM, Curiel-Lewandrowski C, et al. Addressing the knowledge gap in clinical recommendations for management and complete excision of clinically atypical nevi/dysplastic nevi: Pigmented Lesion Subcommittee consensus statement. JAMA Dermatol. 2015;151(2):212–218. [DOI] [PubMed] [Google Scholar]
- 12.Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65(5):1032–1047. [DOI] [PubMed] [Google Scholar]
- 13.Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network. 2013;11(4):395–407. [DOI] [PubMed] [Google Scholar]
- 14.Piepkorn MW, Barnhill RL, Elder DE, et al. The MPATH-Dx reporting schema for melanocytic proliferations and melanoma. J Am Acad Dermatol. 2014;70(1):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onega T, Reisch L, Frederick P, et al. Use of Digital Whole Slide Imaging in Dermatopathology. J Digit Imaging. 2016;29(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carney PA, Reisch LM, Piepkorn MW, et al. Achieving consensus for the histopathologic diagnosis of melanocytic lesions: use of the modified Delphi method. J Cutan Pathol. 2016;43(10):830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalkey NC BB, Cochran N. . The Delphi Method, III. Use of Self Ratings to Improve Group Estimates. Rand Corp. 1969. [Google Scholar]
- 18.Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2013;69(6):1049–1050. [DOI] [PubMed] [Google Scholar]
- 19.Choudhry NK, Fletcher RH, Soumerai SB. Is an Older, More Experienced Doctor a Better Doctor? Annals of Internal Medicine. 2005;142:260–273. [DOI] [PubMed] [Google Scholar]
- 20.Colloby PS, West KP, Fletcher A. Observer variation in the measurement of Breslow depth and Clark’s level in thin cutaneous malignant melanoma. The Journal of pathology. 1991;163(3):245–250. [DOI] [PubMed] [Google Scholar]
- 21.Heenan PJ, Matz LR, Blackwell JB, et al. Inter-observer variation between pathologists in the classification of cutaneous malignant melanoma in western Australia. Histopathology. 1984;8(5):717–729. [DOI] [PubMed] [Google Scholar]
- 22.Boiko PE, Piepkorn MW. Reliability of skin biopsy pathology. J Am Board Fam Pract. 1994;7(5):371–374. [PubMed] [Google Scholar]
- 23.Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30(5):513–520. [DOI] [PubMed] [Google Scholar]
- 24.Cook MG, Clarke TJ, Humphreys S, et al. The evaluation of diagnostic and prognostic criteria and the terminology of thin cutaneous malignant melanoma by the CRC Melanoma Pathology Panel. Histopathology. 1996;28(6):497–512. [DOI] [PubMed] [Google Scholar]
- 25.Krieger N, Hiatt RA, Sagebiel RW, Clark WH Jr., Mihm MC Jr. Inter-observer variability among pathologists’ evaluation of malignant melanoma: effects upon an analytic study. Journal of clinical epidemiology. 1994;47(8):897–902. [DOI] [PubMed] [Google Scholar]
- 26.Piepkorn MW, Barnhill RL, Cannon-Albright LA, et al. A multiobserver, population-based analysis of histologic dysplasia in melanocytic nevi. J Am Acad Dermatol. 1994;30(5 Pt 1):707–714. [DOI] [PubMed] [Google Scholar]
- 27.Spatz A, Cook MG, Elder DE, Piepkorn M, Ruiter DJ, Barnhill RL. Interobserver reproducibility of ulceration assessment in primary cutaneous melanomas. European Journal of Cancer. 2003;39(13):1861–1865. [DOI] [PubMed] [Google Scholar]
- 28.Meyer LJ, Piepkorn M, Goldgar DE, et al. Interobserver concordance in discriminating clinical atypia of melanocytic nevi, and correlations with histologic atypia. J Am Acad Dermatol. 1996;34(4):618–625. [DOI] [PubMed] [Google Scholar]
- 29.Braun RP, Gutkowicz-Krusin D, Rabinovitz H, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the ‘gold standard’? Dermatology (Basel, Switzerland). 2012;224(1):51–58. [DOI] [PubMed] [Google Scholar]
- 30.Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38(7):934–940. [DOI] [PubMed] [Google Scholar]
- 31.Carli P, De Giorgi V, Naldi L, Dosi G. Reliability and inter-observer agreement of dermoscopic diagnosis of melanoma and melanocytic naevi. Dermoscopy Panel. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 1998;7(5):397–402. [DOI] [PubMed] [Google Scholar]