ABSTRACT
Progresses in medical care of severe kidney disease and congenital anomalies of kidney and urinary tract make it possible for a higher percentage of young renal failure patients to survive and enter adulthood. There is thus an increasing need to focus on the long-term effects of severely reduced kidney function early in life. Cardiovascular changes are known to contribute considerably in adulthood to the severe complications of renal failure. In young chronic kidney disease patients, there is limited knowledge of subclinical cardiovascular disease. In this issue of Clinical Kidney Journal, Lalayiannis et al. describe significant structural and functional cardiovascular changes in a young cohort of kidney failure patients with glomerular filtration rate <30 mL/min/1.73 m2. Among the 100 patients between 5 and 30 years of age included in the study, 84 presented with signs of cardiovascular disease. There is a need for long-term follow-up data on cardiovascular consequences of renal failure early in life and evaluation of prophylactic and therapeutic measures that can ameliorate the overall prognosis for these patients. We look forward to planned future long-term data from this cohort as well as increased focus in general on cardiovascular changes in young renal failure patients.
Keywords: cardiorenal disease, cardiovascular disease, child, chronic kidney disease, dialysis, young adults
Chronic kidney disease is a systemic disease influencing many organ systems with great clinical implications in the long run [1]. The impact of chronic kidney disease on cardiovascular disease has been shown to be major [2], and it is recommended to start early cardiovascular prophylaxis in chronic kidney disease patients [3].
Lalayiannis et al. present in this issue of Clinical Kidney Journal significant evidence of subclinical cardiovascular disease in a young renal failure cohort [4]. The findings are based on thorough cardiovascular examination of 100 young kidney failure patients (chronic kidney disease stages 4 and 5) including patients on dialysis. The structural measures performed were carotid intima media thickness, coronary artery calcification, left ventricular mass index and left ventricular geometry evaluation through relative wall thickness. The functional measures consisted of carotid ability to distend with each cardiac cycle, carotid femoral pulse wave velocity, pulse wave augmentation and carotid dilatation, all related to height and age.
Seventy-nine of the patients were children and adolescents (5–18 years of age), and 21 were young adults (19–29 years of age). All adult patients as well as 73% of the children/adolescents were on dialysis. Eighty-four percent were shown to have a minimum of one cardiovascular abnormality—either structural or functional. Having more than one structural abnormality resulted in 4.5-fold increased odds of having multiple functional abnormalities.
These findings of significant preclinical cardiovascular disease in such a young cohort underline the importance of preventive measures to delay cardiovascular complications as much as possible.
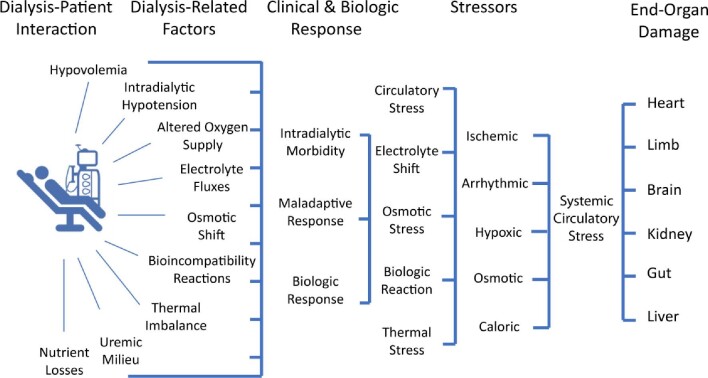
The seventy-seven patients that were on dialysis (5–29 years of age) were compared with the younger group of patients with renal failure without dialysis (5–18 years of age). Higher age-adjusted functional cardiovascular disease was found in the dialysis group. Dialysis per se was independently associated with reduced distensibility. End-stage renal failure treatment with dialysis with its fluctuations in volume status, blood pressure and electrolyte levels as well as osmotic shifts may well have induced systemic damage, and there is a need to develop more cardioprotective and better-tolerated haemodialysis [5]. Potential adverse effects of haemodialysis promoting end-organ damage are depicted in Figure 1.
FIGURE 1:
Dialysis-induced systemic stress acting as disease modifier and resulting in a multiorgan injury superimposed on preexistent comorbidities and affecting outcomes. From Canaud et al. [5].
The years spent with estimated glomerular filtration rate below 30 mL/min/1.73 m2 was an independent predictor of vessel stiffness underlining the cumulative damage to the cardiovascular system as a result of prolonged exposure to the uraemic milieu. The carotid femoral pulse wave velocity as a measure of aortic stiffness is known as an independent negative prognostic factor in patients with chronic kidney disease [6].
There are limited data concerning clinical cardiovascular consequences of phosphate-lowering agents [7]. Future measurements of the serum calcification propensity in future studies in young renal failure patients would be of great interest, a method that represents a functional nanoparticle-based in vitro test, which time-dependently determines the calcification propensity of patient serum also in children with chronic kidney failure undergoing haemodialysis as recently published by Kakajiwala et al. [8]. Serum calcification propensity has the potential to represent a modifiable risk factor for vascular calcification. In future studies a healthy control group would also have the potential to strengthen the findings.
The present study underscores the notion that chronic kidney disease patients generally demonstrate a premature aging phenotype because even children on dialysis may develop accelerated vascular calcification with arterial stiffening, and young renal failure patients can suffer from a cardiovascular mortality to a similar extent to very old subjects of the general population [9]. In accordance, clinically relevant peripheral artery disease was also shown to be increased in chronic kidney disease patients later in adulthood [10, 11].
Consequently, there is an increasing claim of the need for a cardionephrology subspecialty, which is also supported by the fact that partly conflicting pharmaceutical and clinical considerations are existed in the prevention of cardiovascular complications in chronic kidney disease patients [12]. Burlacu et al. underline the importance of good cooperation between cardiologist and nephrologist for the best of each patient, to more agree to disagree [13].
There is a need for awareness concerning the potential renal complications of cardiac diagnostic procedures like contrast use in catheterization and cardiac MRI, and valuable guidelines are in place to reduce the potential complications [14, 15]. It is also important to be aware of the impact immunosuppressive treatment may have, with the potential to aggravate cardiovascular disease also in young kidney transplanted patients [16]. A well-planned and carefully executed transition phase from paediatric to adult medical care needs to be present in all renal units to avoid problems with compliance and additional aggravation of the medical condition [17].
Long-term follow-up data on cardiovascular functional and structural consequences of renal failure early in life are of great importance, and there is a need for evaluation of prophylactic and therapeutic measures in this patient group. Lalayiannis et al.’s planned future long-term data of their cohort are welcomed as well as a general increased focus on cardiovascular changes in young renal failure patients.
Contributor Information
Camilla Tøndel, Department of Pediatrics, Haukeland University Hospital, Bergen, Norway; Renal Research Group, Department of Clinical Medicine, University of Bergen, Bergen, Norway.
Hans-Peter Marti, Renal Research Group, Department of Clinical Medicine, University of Bergen, Bergen, Norway; Renal Unit, Department of Medicine, Haukeland University Hospital, Bergen, Norway.
CONFLICT OF INTEREST STATEMENT
CT is a member of the CKJ editorial board.
FUNDING
None.
REFERENCES
- 1. Zoccali C, Vanholder R, Massy ZA et al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358 [DOI] [PubMed] [Google Scholar]
- 2. Matsushita K, van der Velde M, Astor BC et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA 2019; 322: 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lalayiannis A, Ferro CJ, Wheeler D et al. The burden of subclinical cardiovascular disease in children and young adults with CKD and on dialysis. Clin Kidn J, sfab168, 10.1093/ckj/sfab168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canaud B, Kooman JP, Selby NM et al. Dialysis-induced cardiovascular and multiorgan morbidity. Kidney Int Rep 2020; 5: 1856–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Townsend RR, Anderson AH, Chirinos JA et al. Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC study (Chronic Renal Insufficiency Cohort). Hypertension 2018; 71: 1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lioufas N, Pascoe E, Hawley C et al. Systematic review and meta-analyses of effects of phosphate-lowering agents in non-dialysis chronic kidney disease. J Am Soc Nephrol 2021: ASN.2021040554. doi: 10.1681/ASN.2021040554 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kakajiwala A, Pasch A, Rogers R et al. Serum calcification propensity in children on chronic hemodialysis. Kidney Int Rep 2020; 5: 1528–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shanahan CM. Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol 2013; 9: 661–670 [DOI] [PubMed] [Google Scholar]
- 10. Matsushita K, Ballew SH, Coresh J et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2017; 5: 718–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hishida M, Menez S, Matsushita K. Peripheral artery disease in CKD: anatomically peripheral but clinically central. Am J Kidney Dis 2020; 75: 687–689 [DOI] [PubMed] [Google Scholar]
- 12. Díez J, Ortiz A. The need for a cardionephrology subspecialty. Clin Kidney J 2021; 14: 1491–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burlacu A, McCullough PA, Covic A. Cardionephrology from the point of view of the cardiologist: no more agree to disagree-getting to ‘yes’ for every patient. Clin Kidney J 2021; 14: 1995–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ronco F, Tarantini G, McCullough PA. Contrast induced acute kidney injury in interventional cardiology: an update and key guidance for clinicians. Rev Cardiovasc Med 2020; 21: 9–23 [DOI] [PubMed] [Google Scholar]
- 15. Weinreb JC, Rodby RA, Yee J et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med 2020; 3: 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rangaswami J, Mathew RO, Parasuraman R et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant 2019; 34: 760–773 [DOI] [PubMed] [Google Scholar]
- 17. Diaz-Gonzalez de Ferris ME, Ferris MT, Filler G. Transition from paediatric to adult-focused care: unresolved issues. Nat Rev Nephrol 2021; 17: 705–706 [DOI] [PubMed] [Google Scholar]