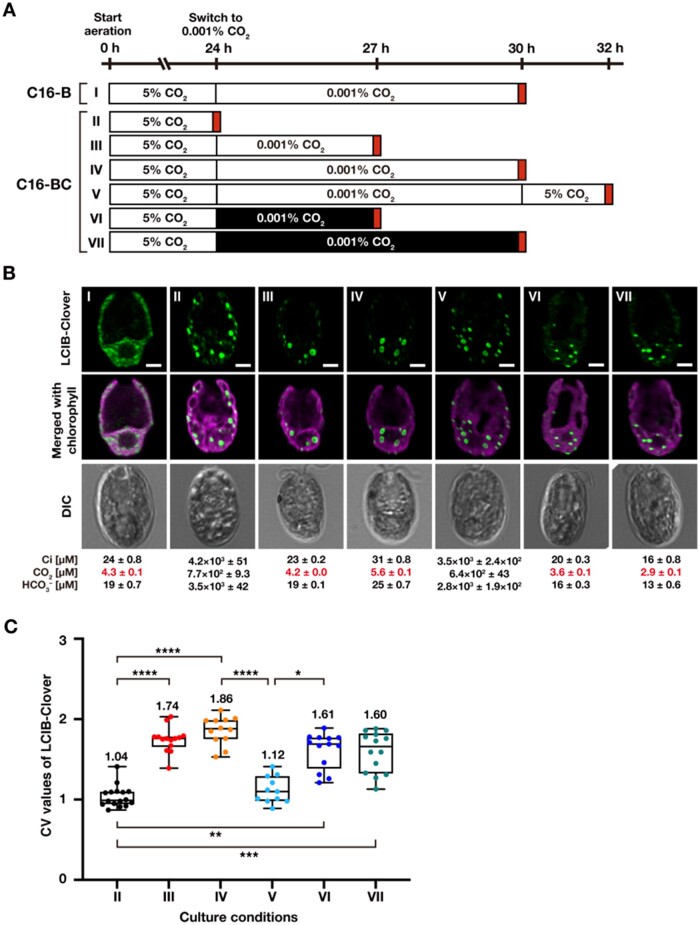
Figure 6.
The requirement of LCIC for the LCIB-Clover migration and relocation. A, Schematic of the liquid culture conditions. C16-B cells were cultured in medium aerated with 5% CO2 illuminated at 120 µmol photons m−2 s−1 (white box) for 24 h, which was replaced with the fresh medium aerated with 0.001% CO2 for 6 h (indicated as I to the left of the white box). C16-BC cells were cultured in medium aerated with 5% CO2 for 24 h (II), which was replaced with the fresh medium aerated with 0.001% CO2 for 3 h (III) or 6 h (IV) in the light or for 3 h (VI) or 6 h (VII) in the dark (black box). After aeration with 0.001% CO2 for 6 h, the concentration of aerated CO2 was switched to 5% for 2 h (V). Red boxes indicate the time of observation. For all culture conditions, the pH of the medium is 7.0. B, Representative LCIB-Clover fluorescence images of C16-B and C16-BC cells. Roman numerals correspond to the culture conditions indicated in A. Ci concentrations in the culture medium were measured using gas chromatography, and calculated CO2 and concentrations are shown below the images. CO2 concentrations shown in red indicate VLC (<7 µM CO2) conditions. DIC, differential interference contrast image. Scale bars: 2 μm. C, Quantification of localization patterns of LCIB-Clover. Roman numerals correspond to the culture conditions indicated in A. The CV value of the fluorescence intensity was calculated in each cell to quantify LCIB-Clover localization. The median values derived from the analysis of C16-BC cells are represented with error bars depicting the interquartile range (n = 11–16). Dunn’s multiple comparisons test was used to assess the statistical significance of LCIB-Clover localization between the different conditions. *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001; ****P-value < 0.0001, Kruskal–Wallis test with Dunn’s multiple comparison.