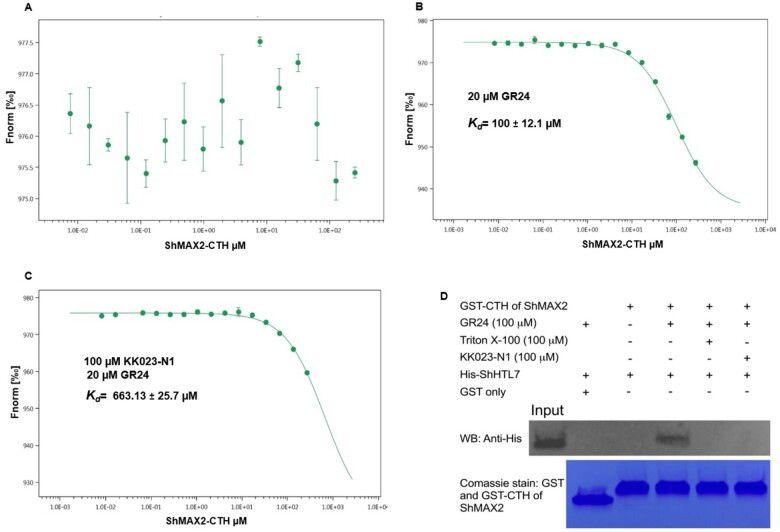
Figure 5.
Determination of Kd by MST for the interaction between ShHTL7 and ShMAX2-CTH. A, The binding affinity between 20 nM of labeled ShHTL7 and ShMAX2-CTH was analyzed in the buffer and no direct interaction was detected between the two proteins in the absence of GR24. B, Addition of 20 µM of GR24 appeared very active to induce the ShHTL7–ShMAX2–CTH interaction. C, The binding affinity shifted upon the addition of KK023-N1 with a dissociation constant Kd = 663.13 ± 25.7 µM, indicating that KK023-N1 is blocking the GR24-dependent interaction between ShHTL7–ShMAX2–CTH. Data are the means ± sd (n = 3). D, GST pull down of His-ShHTL7 by CTH of ShMAX2 in the presence of GR24, Triton X-100, and KK023-N1.