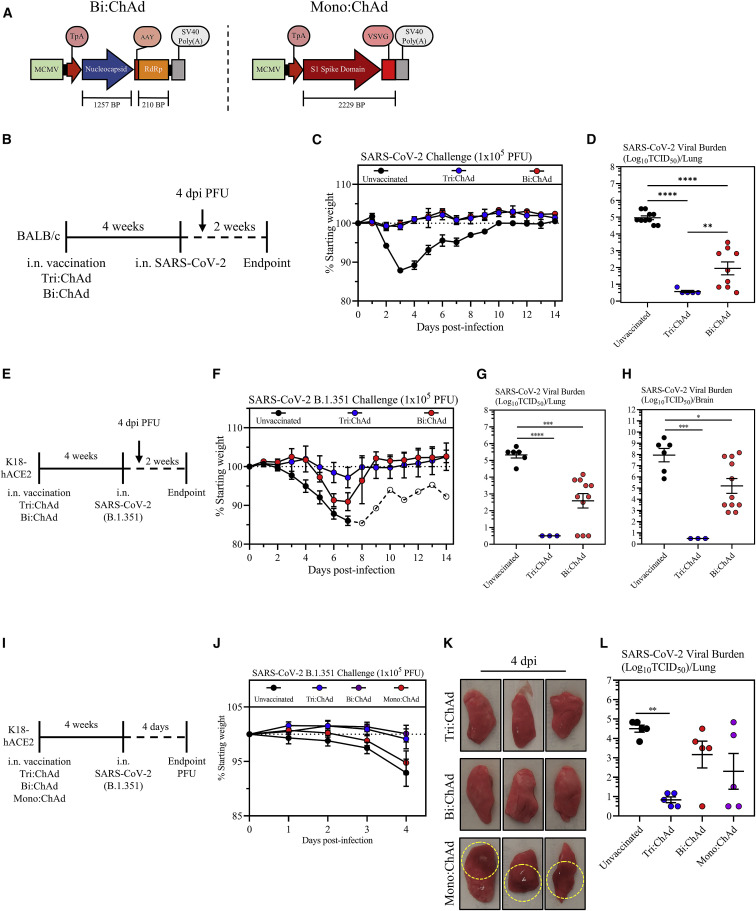
Figure 7.
Comparison of protective efficacy of ChAd-vectored trivalent vaccine with its bi-valent and mono-valent counterparts
(A) Transgene cassette diagrams for bi-valent (Bi:ChAd, left) and mono-valent (Mono:ChAd, right) ChAd vaccines.
(B) Experimental schema.
(C) Changes in body weight over 2 weeks post-SARS-CoV-2 infection.
(D) Viral burden (Log10TCID50) in the lung at 4 days post-SARS-CoV-2 MA10 infection.
(E) Experimental schema.
(F) Changes in body weight over 2 weeks post-SARS-CoV-2 B.1.351 infection (open circles: 1 surviving animal).
(G) Viral burden (Log10TCID50) in the lung at 4 days post-B.1.351 infection.
(H) Viral burden (Log10TCID50) in the brain at 4 days post-B.1.351 infection.
(I) Experimental schema.
(J) Changes in body weight over 2 weeks post-SARS-CoV-2 B.1.351 infection.
(K) Gross pathological changes from the lungs of vaccinated mice at 4 days post-infection. Hashed circles encompass areas of visible lung damage.
(L) Viral burden (Log10TCID50) in the lung at 4 days post-B.1.351 infection.
Data presented in (C, D, F–H, J, and L) represent mean ± SEM. Statistical analysis for (D, G, H, and L) were one-way ANOVA with Tukey’s multiple comparisons test. Data in (C, D, F, G, and H) are pooled from 2 independent experiments, n = 3–11 mice/group. Data in (J–L) are representative of 1 experiment, n = 5 mice/group. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.