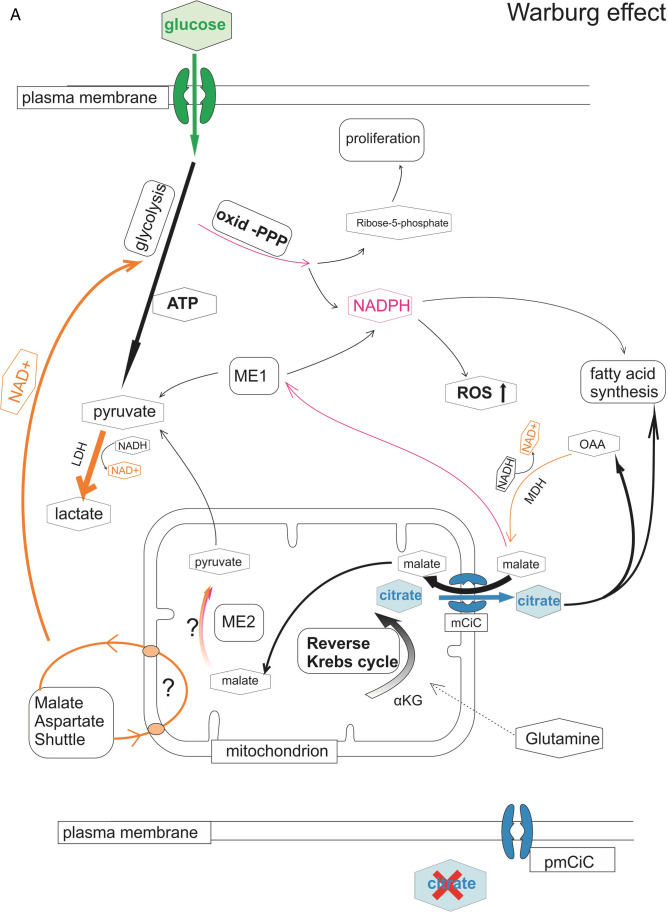
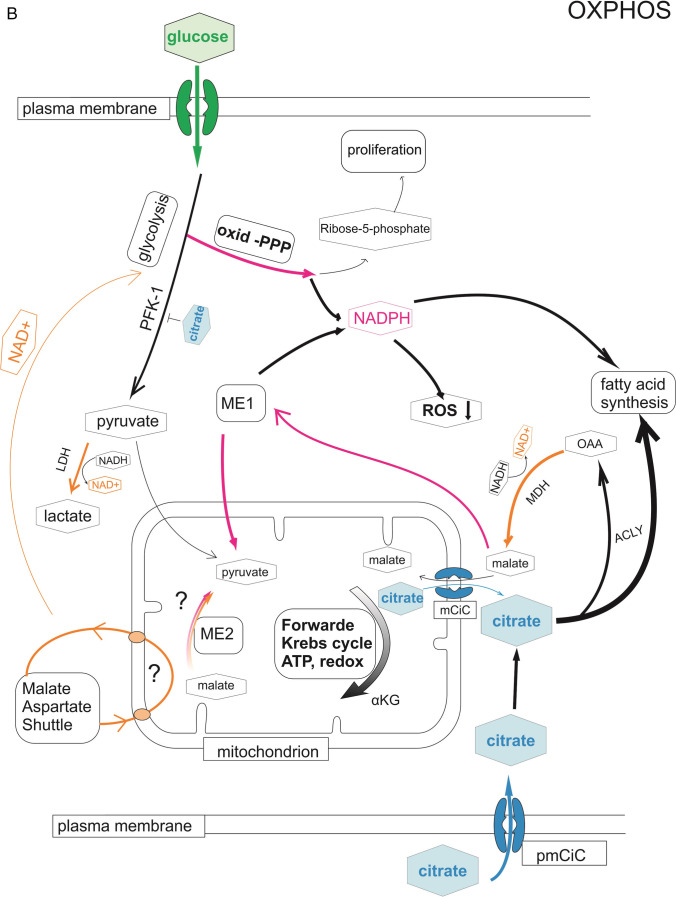
Fig. 1.
Involvement of citrate in Warburg effect versus OXPHOS. Diagram summarising the hypothesis that cancer cells potentially use different metabolic pathways in the presence or absence of extracellular citrate supported by the data discussed in the present review. (A) In the absence of extracellular citrate cancer cells need to synthesise excess citrate intracellularly in the process of the reverse Krebs cycle. In order to keep redox balance under these conditions, several different pathways to account for the needed NAD+/NADH and NADP+/NADPH are used. NAD+ necessary to support the process of glycolysis will be supplied mainly by LDH converting pyruvate into lactate. Because a part of the glycolysis intermediates is used to increase biomass, the action of LDH alone has been determined not to be sufficient. To increase NAD+ levels, citrate coming from mitochondria into the cytoplasm can be converted into oxaloacetate, a by-product of ATP-citrate lyase. MDH1 can further metabolise oxaloacetate into malate which can be then transported into mitochondria in exchange to citrate and support activity of the MDH2 (part of MAS) and ME2 contributing to the NAD+ pool. Alternatively, malate coming from the reaction of MDH1can also be further metabolised by ME1 to support NADPH level. Pyruvate, the product of ME1 can be further metabolised by LDH and supplying NAD+ for glycolysis. Under conditions without extracellular citrate oxidative PPP activity is likely to be decreased as it competes for G6P with glycolysis. Decreased PPP activity would lead to decreased NADPH level resulting in increased ROS. (B) On the other hand, when extracellular citrate is available, its uptake can support fatty acid synthesis in the cytoplasm allowing for the forward Krebs cycle and ATP synthesis in mitochondria. Decreased glycolytic activity will result in decreased need of NAD+ which might reduce the need for MAS activity allowing for undisturbed Krebs cycle. Decreased use of glycolysis will allow for increased NADPH supply through PPP leading to decreased ROS levels. Use of oxidative PPP will be also supported by increased levels of cytosolic citrate inhibiting phosphofructokinase (PFK-1). Increased cytosolic levels will allow for increased fatty acid synthesis. Oxaloacetate produced through the action of ATP citrate lyase can be further converted to malate, increase NADPH levels through ME1 and further supply pyruvate to mitochondria. Pathways contributing to NAD+ synthesis are depicted in orange, to NADPH in dark pink. Thickness of the lines represents activity level of the particular metabolic pathways. α-KG, α-ketoglutarate; ACLY, ATP citrate lyase; LDH, lactate dehydrogenase; ME1, 2, malic enzyme; MDH, malate dehydrogenase; MAS, malate aspartate shuttle, oxid-PPP, PPP oxidative branch; PFK-1, phosphofructokinase-1