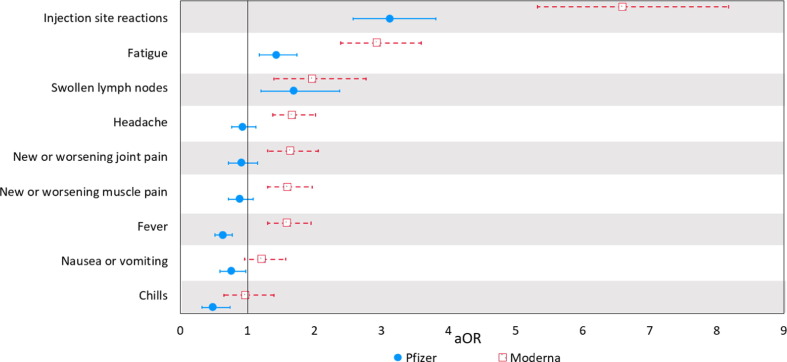
Fig. 1.
Odds of COVID-19 vaccine side-effects from Moderna and Pfizer vaccines compared to the J&J vaccine (Adjusted Odds Ratios (aOR) and 95% Confidence Intervals (CI)).
Note: For two-dose vaccine series, data include side effects reported after either first or booster dose. Adjusted ORs are controlled for by age, education, ethnicity, race (White, Black/African American, Other), BMI, receipt of influenza vaccine, gender (female vs. non-female), smoking status, and medical conditions (depression, anxiety, insomnia or trouble sleeping, cardiovascular disease, kidney disease, hypertension, diabetes, and lung disease. Due to the possibility of quasi-complete separation of data points because of small sample sizes in the referent group (J&J) experiencing severe allergic reactions, dizziness, and diarrhea, we do not present results for these outcomes.