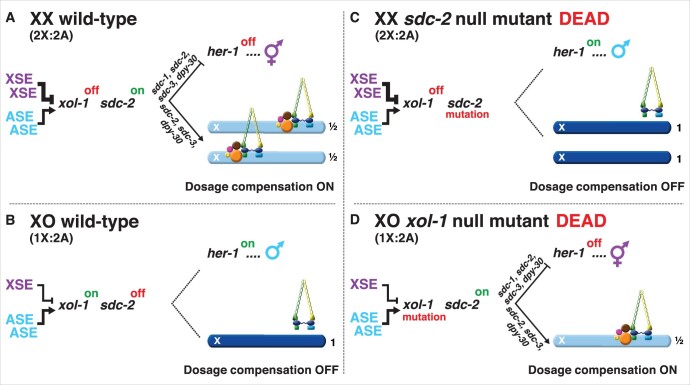
Figure 2.
Overview of the X:A signal and the regulatory hierarchy that controls nematode sex determination and dosage compensation. (A, B) In wild-type animals, the X:A signal that determines sexual fate is a competition between a set of genes on X called XSEs that represses their direct gene target xol-1 (XO lethal) in a cumulative dose-dependent manner via transcriptional and post-transcriptional mechanisms and a set of genes on autosomes called ASEs that stimulate xol-1 transcription in a cumulative dose-dependent manner. xol-1 is the master sex-determination switch gene that must be activated in XO animals to set the male fate and must be repressed in XX animals to permit the hermaphrodite fate. (A) Two doses of XSEs in diploid XX animals win out and repress xol-1, but (B) the single dose of XSEs in diploid XO animals does not turn xol-1 off. (B) xol-1 triggers male sexual development in wild-type XO animals by repressing the feminizing switch gene sdc-2 (sex determination and dosage compensation). (A) Together with sdc-1, sdc-3, and dpy-30, the sdc-2 gene induces hermaphrodite sexual development in XX animals by repressing the male sex-determining gene her-1. Together with sdc-3 and dpy-30, sdc-2 triggers binding of a dosage compensation complex (DCC) onto both hermaphrodite X chromosomes to repress gene expression by half. sdc-1 is essential for DCC activity, but not for loading of the DCC onto X. The DCC is a condensin complex that restructures the topology of X. (C) sdc-2 mutations kill XX animals by prevent the DCC from binding to X chromosomes, resulting in overexpression of X-linked genes. The mutations also masculinize XX animals, because her-1 is not repressed. (D) Loss-of-function xol-1 mutations enable sdc-2 to be active and permit the DCC to bind the single male X, thereby killing XO animals from reduced X-chromosome expression. The dying xol-1 XO mutant animals are feminized because her-1 is repressed. Hence, mutations that disrupt elements of the X:A signal itself transform sexual fate, but also kill due to altered X-chromosome gene expression.