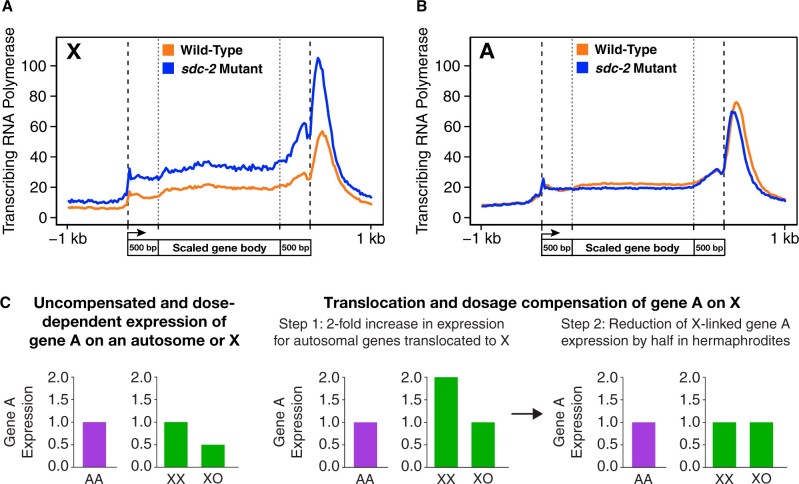
Figure 8.
Plausible strategy for dosage compensation and for balancing gene expression between X chromosomes and autosomes. (A) Evidence that the DCC controls gene expression by limiting RNA polymerase recruitment to promoters. A uniform increase in transcriptionally engaged RNA polymerase (1.7-fold) across the length of X-linked genes, from promoters to 3′ ends, in response to disruption of dosage compensation implicates reduction of RNA polymerase recruitment to X-linked promoters as a plausible mechanism of dosage compensation. Levels of transcriptionally engaged RNA polymerase were measured by global run-on sequencing experiments. The figure shows metagene analysis comparing levels of transcriptionally engaged RNA polymerase from wild-type control embryos and sdc-2 mutant embryos. All genes are depicted by the convention that 5′ ends (−1 kb to + 500 bp of the transcript start sites) and 3′ ends (500 bp upstream to 1 kb downstream of the 3′ end) are not scaled but all gene bodies are scaled to 2 kb. (B) Levels of transcriptionally engaged RNA polymerase on genes of autosomes are slightly decreased in sdc-2 mutant vs wild-type control embryos, potentially because the limited amount of RNA polymerase in the cell is recruited to the numerous nondosage-compensated X-linked genes in the sdc-2 mutant. Analysis was conducted and depicted as in (A). Average levels of transcriptionally engaged polymerase are similar between X and autosomal genes (Kruesi et al. 2013). (B) Balancing gene expression between X chromosomes and autosomes. Recognizing that the reduction of X-chromosome gene expression in XX females (or hermaphrodites) as a mechanism for dosage compensation between sexes might create a deleterious reduction in X-chromosome products for both sexes, Susumo Ohno proposed a two-step mechanism for the recruitment of autosomal genes to X chromosomes and the concomitant regulation of X-linked gene expression (Ohno 1967). During the evolution of X chromosomes from autosomes and the connected establishment of X-chromosome dosage compensation, a mechanism would arise to increase the expression level of autosomal genes translocating to X by twofold in both sexes (step 1). This upregulation of X expression would make expression from the male X equal to that of the ancestral autosomes but would cause a twofold overexpression of X-linked genes in females (or hermaphrodites) relative to the ancestral autosomes. The overexpression in females (or hermaphrodites) would then be offset by an X-chromosome dosage compensation process that reduced X expression in females (or hermaphrodites), thereby balancing X expression between sexes, as well as balancing expression between female (or hermaphrodite) X chromosomes and the ancestral autosomes (step 2). Evidence from gene expression studies supports this model for C. elegans.