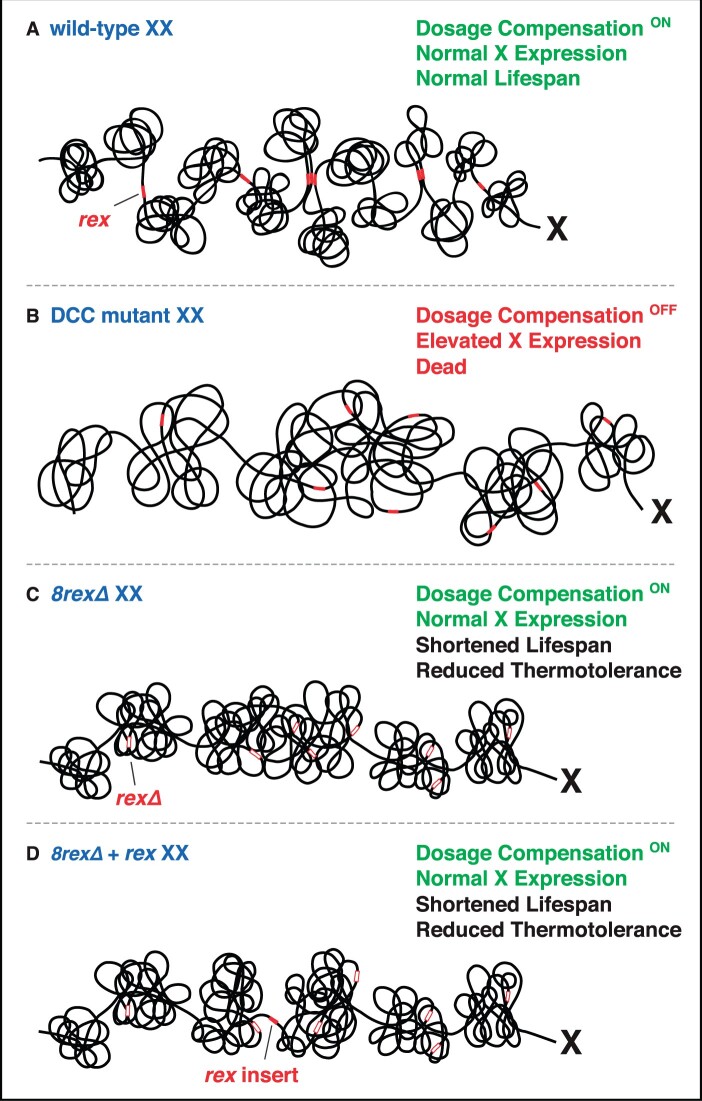
Figure 9.
X-chromosome domain architecture established by DCC binding to rex sites regulates C. elegans lifespan but not dosage compensation. (A) DCC binding at each of eight high-occupancy rex sites (red rectangles) results in a TAD boundary on hermaphrodite X chromosomes. Median lifespan of wild-type XX hermaphrodites is 23 days. (B) The sdc-2 XX mutant animals lack all DCC-dependent TAD boundaries on X, and embryos exhibit overexpression of X-linked genes and die. X-chromosome volume is expanded. (C, D) A single rex deletion at each boundary disrupts the boundary (C) and a single rex insertion (D) creates a new boundary, demonstrating that a high-occupancy rex site on X can be both necessary and sufficient to define a DCC-dependent boundary location. In contrast to sdc-2 mutant embryos, 8rexΔ mutant embryos exhibited no changes in X volume or X expression, and 8rexΔ adults lack dosage-compensation mutant phenotypes. Hence, TAD boundaries are neither the cause nor consequence of DCC-mediated gene repression. Abrogating TAD structure did, however, reduce thermotolerance, accelerate aging, and shorten lifespan by 20% (C), implicating chromosome architecture in stress responses and aging. Inserting a rex site in a new location in 8rexΔ mutants failed to suppress the reduced lifespan or reduced thermotolerance (D).