Abstract
In recent years, quinoline-based fibroblast activation protein (FAP) inhibitors (FAPI) have shown promising results in the diagnosis of cancer and several other diseases, making them the hotspot of much productive research. This review summarizes the literature for the state-of-the-art FAPI-PET imaging for cancer diagnosis compared with fluorodeoxyglucose (FDG)-PET. We also summarize the use of FAPI-PET for therapeutic regimen improvement and fibroblast activation protein (FAP)-targeted molecule modification strategies, as well as preliminary clinical studies regarding FAP-targeted radionuclide therapy. Our qualitative summary of the literature to date can inform future research directions, medical guidelines, and optimal clinical decision-making.
Keywords: cancer-associated fibroblasts (CAF), fibroblast activation protein (FAP), targeted radionuclide therapy, PET/CT, cancer management
Introduction
Approximately 19.3 million new cancer diagnoses and approximately 10.0 million cancer deaths occurred worldwide in 2020, according to estimates from Global Cancer Statistics 1. Cancers develop in complex environments composed of tumor cells and the surrounding stroma. The “seed and soil” theory emphasized the interactive importance of both components as early as 1889 2. However, diagnostic and therapeutic paradigms have predominantly targeted only tumor cells.
The widespread application of immunotherapy in clinical trials has led to increased research attention being paid to the tumor microenvironment (TME). The tumor stroma or TME comprises all the noncancer components in the tumor tissue, including cancer-associated fibroblasts (CAFs), the extracellular matrix (ECM), various types of immune cells, and intertwined blood vessels. The TME can develop an immunosuppressive niche in which tumor cells are protected from conventional therapies, resulting in treatment failure 3.
CAFs are among the most abundant components of the TME in solid tumors 4. However, CAFs are heterogeneous cells with both tumor-promoting and tumor-suppressive effects observed in different situations 5. CAFs model and remodel the ECM structure, which can become a physical barrier against the infiltration of immunocytes, which have a killer function, or a structural scaffold for intercellular interaction between tumor cells and non-tumor cells in the TME, thereby regulating tumor initiation, neovascularization, and metastasis 6. On the other hand, CAFs can secrete multiple chemokines and cytokines, such as transforming growth factor-β (TGFβ), CC-chemokine ligand 2 (CCL2), and interleukin-6 (IL-6), in order to recruit immunocytes with inhibitory functions in the tumor stroma, thereby facilitating immune evasion 7.
CAFs have several biological markers, including α-smooth muscle actin, fibroblast activation protein (FAP), and platelet-derived growth factor receptor-β (PDGFRβ). FAP is a type II integral membrane glycoprotein belonging to the serine protease family involved in ECM remodeling and fibrogenesis 8. In colorectal cancers, FAP expression is higher at the invasive front than in the tumor center 9.
Although the prognostic value of FAP in cancers has been inconsistent throughout the literature, high expression of FAP has been shown to be an independent poor prognostic marker for outcomes in lung cancer, hepatocellular carcinoma, and colon cancer in studies with large sample sizes (n = 138-449 patients) 10-12. In a murine model, tumor growth could be potentiated by the constitutive expression of FAP, which could, in turn, be meaningfully attenuated by anti-FAP antibodies 13. However, sibrotuzumab (a humanized version of the murine anti-FAP antibody) failed as a treatment regimen in an early phase II trial for metastatic colorectal cancer 14. Although FAP antibodies have shown limited response in tumor therapy, small molecules targeting FAP have attracted increasing attention in the area of tumor theranostics.
Targeting FAP for tumor imaging
FAP, expressed at low levels in healthy tissues, is involved in various pathological conditions and is detected in over 90% of malignant epithelial tumors 8, 15. Thus, molecular imaging (including positron emission tomography [PET] and single-photon emission computed tomography [SPECT]) targeting FAP is a promising diagnostic imaging modality.
A series of quinoline-based FAP inhibitors (FAPIs) was developed by the University Hospital Heidelberg group based on clinical and preclinical research 16-18. The first FAPI variants (FAPI-01 and FAPI-02) were reported in 2018, demonstrating that FAPI-02 has an improved binding affinity to human FAP compared with FAPI-01 16. Impressively, 68Ga-FAPI-02 PET images showed favorable tracer uptake in tumor tissues and low background uptake in normal healthy organs, resulting in high-contrast images in tumor-bearing murine models and three cancer patients 16. Subsequently, FAPI-04 was identified as the best tracer from among 15 modified FAPIs for PET imaging applications, showing a higher tumor uptake in murine xenograft models than FAPI-02 17. Notably, 68Ga-FAPI-04 was evaluated in a cohort of 80 patients presenting with 28 types of tumors (54 primary tumors and 229 metastases). It showed overall intense tracer uptake (with a maximum standard unit value [SUVmax] > 6) and high-contrast images in various highly prevalent cancers, including sarcomas, cholangiocarcinoma, and esophageal, breast, lung, hepatocellular, colorectal, head-neck, ovarian, pancreatic, and prostate cancers 19. To improve the pharmacokinetics of this PET tracer, FAPI-46 was discovered from 15 other FAPI derivatives, and it demonstrated an enhanced tumor-to-background ratio (TBR) compared with FAPI-04 18. Interestingly, 68Ga-FAPI-46 PET/CT imaging acquisition at an early time point (10 min p.i.) had an equivalent lesion detection rate compared with a late time point (60 min p.i.) in a previous study 20.
Since the dodecane tetraacetic acid (DOTA) ligand was used as a chelator, FAPI-04/46 could also be radiolabeled with therapeutic nuclides such as 90Y, 177Lu, and 225Ac. In addition, the NOTA ligand was used in FAPI-74 for labeling with both 18F and 68Ga, showing favorable image contrasts in PET/CT imaging in various cancers 21. Similarly, the chelator bis((1-(2-(tert-butoxy)-2-oxoethyl)1H-imidazol-2-yl) methyl) glycine was applied to [99mTc]Tc-Labeled FAPI tracers, and [99mTc]Tc-FAPI-34 SPECT was performed on two patients with ovarian and pancreatic cancers 22.
It should be noted that increased FAPI uptake has been reported in many non-oncological conditions, including inflammatory lesions, fibrotic disease, trauma, arthritis, degenerative bone disease, immunoglobulin 4 [IgG-4] related diseases, connective tissue disease, and atherosclerosis 23, 24. Thus, imaging interpretation with 68Ga/18F-FAPI PET/CT should be interpreted with caution to avoid misdiagnosis. Interestingly, increased FAPI uptake in non-oncological conditions could open possibilities for broader use of FAPI for corresponding diseases. For example, FAP-specific PET/CT could be used in the discrimination between inflammatory and fibrotic activity in IgG-4 related diseases 25, evaluation of the progression in atherosclerotic plaques 26, and assessment of the disease activity in fibrotic interstitial lung diseases 27, 28.
Comparing 68Ga-FAPI and 18F-FDG uptake in various types of cancer
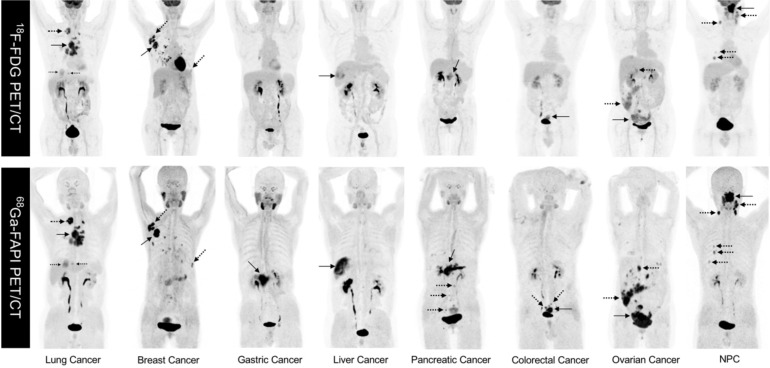
As FAPI is a novel PET tracer in cancer imaging, it is critical to evaluate lesion detection rates and diagnostic efficacy for FAPI compared with 18F-fluorodeoxyglucose (18F-FDG), the dominant tracer in oncology. To the best of our knowledge, Chen et al. conducted the first head-to-head study comparing 68Ga-FAPI-04 and 18F-FDG PET/CT in a cohort of 75 patients (54 patients identified at initial assessment and 21 patients with recurrence detection) with 12 different tumor entities. This prospective study demonstrated that 68Ga-FAPI-04 had a higher sensitivity as compared with 18F-FDG in identifying primary tumors (98.2% vs. 82.1%, P = 0.021), lymph node metastases (86.4% vs. 45.5%, P = 0.004), and bone and visceral metastases (83.8% vs. 59.5%, P = 0.004) 29. However, the limited number of patients harboring each cancer type enrolled in this study did not allow for a subgroup comparison in terms of diagnostic efficacy for the same tumor type. Representative MIP images of both 18-FDG PET/CT and 68Ga-FAPI PET/CT in 8 patients with different types of cancer are shown in Figure 1. Some studies have recently compared the diagnostic efficacy of 68Ga-FAPI and 18F-FDG in various types of tumors.
Figure 1.
Representative comparison of 8 patients with different tumor entities undergoing both 18F-FDG PET and 68Ga-FAPI-04 PET imaging within less than 1 week. Solid arrows indicate primary tumors, while the dotted arrows indicate metastasis lesions. NPC: nasopharyngeal carcinoma.
Head and neck cancer
68Ga-FAPI-04 PET/CT demonstrated improved sensitivity when compared with 18F-FDG PET/CT for cancers of unknown primary origin (CUP), while the sensitivity for detecting primary tumors was comparable in nasopharyngeal carcinoma (NPC), oral squamous cell carcinoma, and Waldeyer's tonsillar ring cancer 30-34. Impressively, 68Ga-FAPI-04 PET/CT pinpointed 39% (7/18) of primary head and neck CUP tumors among primary patients with negative 18F-FDG findings 30. In another cohort of 45 patients with NPC, 68Ga-FAPI-04 PET/CT showed higher radiotracer uptake than 18F-FDG for primary tumors, regional lymph nodes, and distant metastases, resulting in higher sensitivity for the detection of lymph nodes and distant metastases 31. Interestingly, Qin et al. reported that 68Ga-FAPI-04 PET/CT detected a smaller number of positive lymph nodes compared with 18F-FDG PET/CT (the detected number of positive lymph nodes was 48 vs. 100) 32. However, no suspicious FDG-avid lymph nodes were confirmed histologically. This study indicated that 68Ga-FAPI PET/CT might be more specific than 18F-FDG for differentiating reactive lymph nodes from tumor metastatic lymph nodes 35, and it is very likely that the FDG-positive/FAPI-negative lymph nodes are reactive lymph nodes. However, this finding requires validation in future research.
Both studies mentioned above demonstrated that 68Ga-FAPI outperformed 18F-FDG PET/CT in evaluating skull base and intracranial invasion in NPC 31, 32. In a study of 10 patients with oral squamous cell carcinoma (OSCC), both 68Ga-FAPI-04 PET/CT and 18F-FDG PET/CT had comparable sensitivity and specificity for detecting primary tumors (100% vs. 100%) and cervical lymph node metastases (81.3% vs. 87.5%, P = 0.32; 93.3% vs. 81.3%, P = 0.16) 33. However, both radiotracers have relatively high physiological uptakes in the oral mucosa, potentially leading to a compromised target-to-blood pool ratio (TBR) 33. For cancers of Waldeyer's tonsillar ring, 68Ga-FAPI-04 PET/CT showed a higher detection rate in primary tumors but an inferior detection rate in lymph node metastases compared with 18F-FDG PET/CT. However, 68Ga-FAPI-04 PET/CT demonstrated a higher TBR than 18F-FDG PET/CT for detecting primary tumors (10.90 vs. 4.11) 34.
Digestive system cancer
68Ga-FAPI-04 PET/CT demonstrated improved sensitivity in liver, gastric, and pancreatic cancers when compared with 18F-FDG PET/CT 36-45, while the sensitivities of both tracers were comparable in colorectal and esophageal cancers 39, 46. For liver cancer, including hepatocellular carcinoma and intrahepatic cholangiocarcinoma, 68Ga-FAPI-04 has been demonstrated to have a higher sensitivity in detecting primary liver tumors (partly attributed to higher tumor uptake and lower hepatic background uptake as compared with 18F-FDG) as well as extrahepatic metastases 36-38. In gastric cancer, the sensitivity of 68Ga-FAPI-04 PET/CT was higher than that of 18F-FDG PET/CT in detecting primary tumors, lymph nodes, and distant metastases 39-42, 45, especially for signet-ring cell carcinoma and mucinous adenocarcinoma.
In gastric cancer, Pang et al. and Qin et al. reported that the FAPI-derived SUVmax in primary and metastatic lesions was significantly higher than the FDG-derived SUVmax 39, 40, while Jiang et al. and Kuten et al. reported that FAPI-derived TBR was higher than FDG-derived TBR without accompanying differences in the SUVmax 41, 42. In colorectal cancer, 68Ga-FAPI-04 PET/CT has been demonstrated to have equal sensitivity in primary tumor detection compared to 18F-FDG PET/CT (6/6, 100%), although the FAPI-derived SUVmax was statistically significantly higher than the FDG-derived SUVmax in a previous study 39.
Although 68Ga-FAPI-04 has significantly higher uptake compared with 18F-FDG in esophageal cancer, their sensitivity in detecting primary tumors was shown to be comparable 46. Pancreatic tumors are characterized by intense stromal desmoplastic reactions surrounding cancer cells, and CAFs are the main effector cells involved in this desmoplastic reaction. As expected, 68Ga-FAPI-04 PET/CT shows higher sensitivity in detecting primary tumors, including lymph nodes, and metastases than 18F-FDG PET/CT in pancreatic cancer, mainly due to the intense 68Ga-FAPI uptake in pancreatic tumor lesions 43. However, non-specific 68Ga-FAPI uptake in tumor-induced pancreatitis should be noted, as intense 68Ga-FAPI uptake is normally observed throughout the whole pancreas in this disease. This phenomenon is frequently observed in tumors located in the head of the pancreas. As a solution, dual-time point 68Ga-FAPI PET/CT (1 h early-point and 3 h late-point scans) may help differentiate pancreatitis from malignancy 43, 44.
Breast cancer
68Ga-FAPI-04 PET/CT was demonstrated to detect a greater number of cancerous lesions with a higher SUVmax for primary tumors, including lymph nodes, and distant metastases compared with 18F-FDG PET/CT in a cohort of 48 breast cancer patients 47. Kömek et al. reported a similar conclusion in a cohort of 20 patients 48.
Lung Adenocarcinoma
Both 18F-FAPI-42 and 18F-FDG had comparable detection rates (100%) for primary tumors in a cohort of 34 patients 49. Moreover, 18F-FAPI-42 showed higher SUVmax compared to 18F-FDG in the lymph nodes, pleura, bones, and other tissue lesions (P < 0.05), as well as better TBR compared to 18F-FDG in brain lesions (9.53 ± 12.07 vs. 1.01 ± 0.49, P < 0.0001). Therefore, 18F-FAPI-42 may have advantages over 18F-FDG for the primary staging of lung adenocarcinoma. However, 18F-FAPI-42 is inferior in detecting brain lesions compared to contrast-enhanced magnetic resonance imaging (CE-MRI) (56 vs. 34, P = 0.002). Interestingly, one recent study demonstrated intense 68Ga-FAPI activity but minimal 18F-FDG uptake in a malignant pulmonary ground-glass opacity (GGO) nodule 50. Further studies are needed to determine the effectiveness of 68Ga-FAPI and 18F-FDG PET/CT for characterizing GGO nodules.
Sarcomas
The detection rates for primary lesions observed with 68Ga-FAPI-46 PET/CT and 18F-FDG PET/CT were equally high (33/43 vs. 35/43) for bone or soft tissue sarcomas. Moreover, 68Ga-FAPI-46 PET/CT allowed for detecting additional distant metastatic lesions that were not detected via 18F-FDG PET/CT in 6/43 patients 51.
Hematological neoplasms
Head-to-head comparison studies of hematological neoplasms are relatively rare compared to studies evaluating solid tumors. Lan et al. reported that 68Ga-FAPI-04 PET/CT demonstrated inferior sensitivity (50.65 vs. 96.75%) and accuracy (51.28 vs. 95.51%, P < 0.001) than 18F-FDG in a subgroup of eight patients with hematological neoplasms, including multiple myeloma and lymphoma 52.
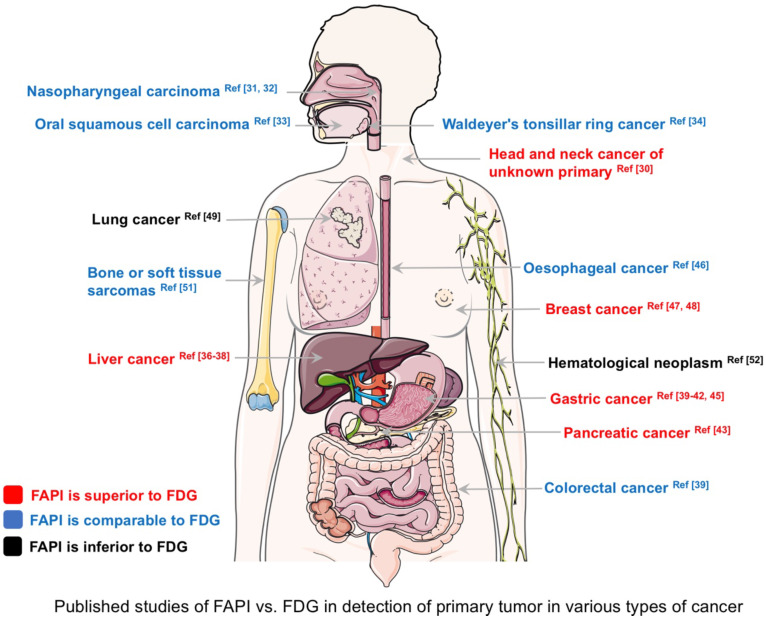
The primary lesion detection rates for 68Ga-FAPI and 18F-FDG PET/CT (based on published data) in various types of cancer are summarized in Figure 2. 68Ga-FAPI PET/CT outperformed 18F-FDG PET/CT in CUP, breast cancer, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, gastric cancer, and pancreatic cancer. In these diseases, 68Ga-FAPI PET/CT may have the potential to replace 18F-FDG in the future. Conversely, 68Ga-FAPI PET/CT was inferior to 18F-FDG PET/CT for diagnosing hematological neoplasms. However, it should be noted that Figure 2 is not based on a systematic literature search of PubMed/MEDLINE and Cochrane Library database analysis, and the number of patients enrolled in previous studies was very small. It should also be noted that the results of Figure 2 only aimed at comparing the detection rate of primary tumors between 18F-FDG and FAPI PET/CT based on existing investigations, including retrospective or smaller prospective studies. For more information, a systematic review was conducted by Treglia et al. to compare radiolabeled FAPI and 18F-FDG PET/CT in oncologic imaging 53. Clear comparability, especially superiority or inferiority, between FAPI vs. FDG should be demonstrated in future studies.
Figure 2.
Published studies comparing fibroblast activation protein inhibitor-positron emission tomography (FAPI) vs. fluorodeoxyglucose positron emission tomography (FDG) in the diagnosis of various types of cancer (detection rate of primary tumors). The corresponding references are presented in the figure.
Overall, we conclude that 68Ga-FAPI PET/CT has comparable or improved diagnostic performance in imaging various cancers compared to 18F-FDG PET/CT, particularly in cancer types that normally show a low-to-moderate 18F-FDG uptake 54, 55. Lower background uptake in normal organs leads to higher TBRs with 68Ga-FAPI when compared with 18F-FDG 56. As stroma components can comprise up to 50-90% of the tumor environment, stroma-targeted PET/CT imaging could be more sensitive than glucose metabolism-based PET imaging in detecting small lesions or lesions with low glucometabolic activity 57. Moreover, 68Ga-FAPI PET/CT provides advantages over 18F-FDG PET/CT because it does not require fasting and imaging acquisition at early time points following injection (i.e., 10-60 min after tracer administration). However, it is too arbitrary to say FAPI PET/CT will replace FDG PET/CT based on the current clinical studies. The Role of FAPI PET in cancer imaging and management should be further explored in larger prospective trials before any conclusion should be reached.
Changes in cancer management according to 68Ga/18F-FAPI PET/CT
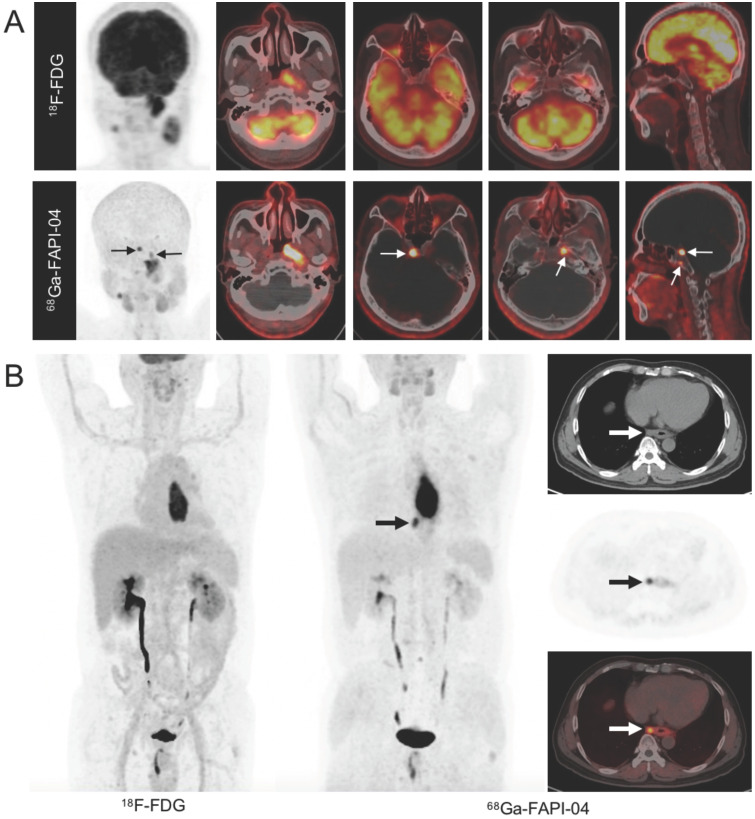
In addition to studies comparing 68Ga-FAPI and 18F-FDG PET/CT for the diagnosis of various types of cancer, several studies have explored the added value of 68Ga-FAPI PET/CT in cancer management compared with standard care of imaging (SCI). In oral squamous cell carcinoma, 68Ga-FAPI-04 PET/CT showed superior sensitivity (81.3% vs. 50.0%) and specificity (93.3% vs. 61.5%) in detecting lymph node metastases compared with CE-MRI 33. Specifically, compared with CE-MRI, 68Ga-FAPI PET/CT upgraded and underestimated the T stage in 4/39 and 2/39 patients with NPC, respectively (Figure 3A) 31. Zhao et al. reported that 68Ga-FAPI-04 PET/CT improved tumor staging in patients with esophageal cancer, compared to contrast-enhanced CT and 18F-FDG PET/CT (Figure 3B) 58. Pang et al. and Röhrich et al. reported that the use of 68Ga-FAPI-04/46 PET/CT led to changes in oncologic management in 1/23 patients and 7/19 patients, respectively, due to the upstaging of the TNM stage as compared with contrast-enhanced computed tomography (CE-CT) 43, 44. Regarding anal canal carcinoma, 68Ga-FAPI-46 PET/CT led to a change in the TNM classification in 3/6 patients as compared with MRI and CT in a previous study (i.e., based on FAPI-positive nodes in two patients with ill-defined results on MRI and FAPI-negative lesions in one patient with suspected pulmonary lesions on CT) 59.
Figure 3.
A. Imaging findings in a 51-year-old treatment-naive male patient with nasopharyngeal carcinoma. 18F-fluorodeoxyglucose (FDG) (upper row) and 68Ga-labeled fibroblast activation protein inhibitor (FAPI) positron emission tomography/computed tomography (PET/CT) (lower row) reveal abnormal activity in the nasopharynx. However, intense 68Ga-FAPI uptake is observed in the skull base (white arrow) along with normal FDG uptake, confirmed by magnetic resonance imaging. The TNM stage was upgraded from T2N2 (FDG-based) to T3N2 (FAPI-based). B. Imaging findings in a 56-year-old treatment-naive male patient with esophageal squamous cell carcinoma. 18F-FDG PET/CT for tumor staging to decide the most proper treatment strategy. Maximum intensity projection (MIP) image 18F-FDG PET/CT reveals an intense FDG-avid mass in the mid-esophagus, while the MIP image of 68Ga-FAPI-04 shows intense uptake of FAPI in the primary tumor and paraesophageal lymph node. This FAPI-positive lymph node, suggestive of nodal metastasis, was later confirmed by histopathology. Tumor staging was upgraded to stage IIIB based on FAPI. Adapted with permission from 31, copyright 2021 Springer, and 58, copyright 2020 Springer.
Optimization of TNM staging via FAPI PET/CT results in improving oncologic management. For example, the course of clinical management was changed in 13 (30%) patients with sarcomas following 68Ga-FAPI-PET in a previous investigation, including major changes (e.g., changes in therapeutic strategy) in seven (16%) patients 51. However, 68Ga-FAPI PET/CT imaging is not always superior to conventional SCI. For example, 68Ga-FAPI-04 PET/CT detected fewer lesions as compared with MRI of the liver (85% [41/ 48] vs. 100% [48/48], P = 0.34) in a cohort of 32 patients with liver cancer 36. Therefore, it is recommended that 68Ga-FAPI PET/CT be used as a tool complementary to 18F-FDG PET/CT and SCI. Additional investigation is required, as current studies investigating the advantages and disadvantages of 68Ga-FAPI PET/CT are highly limited.
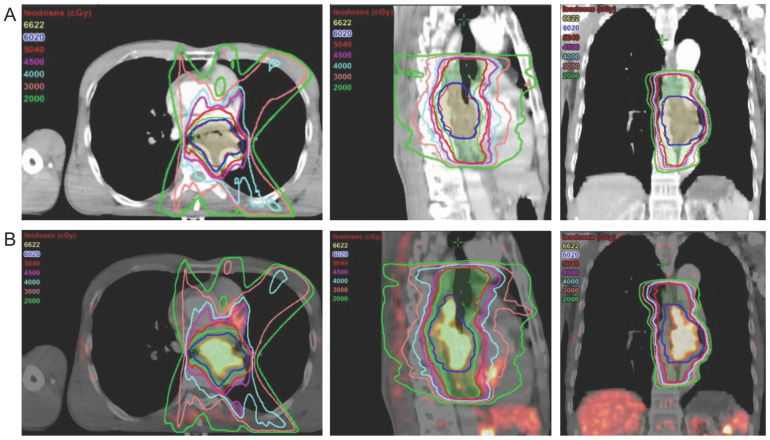
In addition to changes in TNM classification, a clear contour with a favorable TBR improves target volume delineation for radiotherapy. Syed et al. first introduced 68Ga-FAPI PET/CT images in tumor radiotherapy for evaluating gross tumor volume (GTV) contouring 60. Four thresholds (three-, five-, seven- and ten times that of the 68Ga-FAPI uptake in normal tissue surrounding the tumor) were used to generate FAPI-GTV values (FAPI × 3, 5, 7 and 10) in 14 patients with head and neck cancers, which were subsequently compared with GTV values measured conventionally via CE-CT and MRI (CT-GTV). The area covered by FAPI-GTV (FAPI × 3 and 5) was significantly different compared to the area covered by CT-GTV, and the FAPI × 3 threshold was recommended as the best among the imaging modalities 60. Similarly, 18F-FAPI-74 × 3 and 68Ga-FAPI-04 × 2 thresholds were considered optimal with respect to radiotherapy planning for lung cancer and locally recurrent pancreatic cancer 21, 61. Windisch et al. compared FAPI-GTV and MRI-GTV values in glioblastoma and found that the FAPI-GTV values for all thresholds were greater than the MRI-GTV values 62. In addition to using background FAPI uptake, Zhao et al. used demarcations at 20%, 30%, and 40% of the SUVmax as thresholds for FAPI-GTV; their results demonstrated that 68Ga-FAPI-04 × 40% of the SUVmax was ideal for reflecting the actual tumor volumes in esophageal cancer (Figure 4) 46. Interestingly, PET/CT with different FAPI variants (68Ga-FAPI-02, 68Ga-FAPI-46, and 68Ga-FAPI-74) acquired at three time points (10 min, 1 h, and 3 h) with a threshold of 25-35% of the SUVmax was used to delineate three FAPI-GTVs in adenoid cystic carcinomas, all of which were more accurate than CT-GTVs (based on CE-CT and CE-MRI); the 68Ga-FAPI PET/CT images acquired 1 h post-injection were presumed to reflect the ideal time point for contouring 63. Without implementing the FAPI threshold mentioned above, Koerber et al. and Ristau et al. reported that 68Ga-FAPI-04/46 PET/CT improved target volume delineation in 6/6 anal canal carcinoma patients and 6/7 esophageal cancer patients 59, 64.
Figure 4.
Radiation treatment plan for a 57-year-old male patient with lower esophageal cancer based on (A) contrast-enhanced CT (tumor length, 4 cm; GTV volume, 39.32 cm3); and (B) CT + FAPI ×20% (tumor length, 7.5 cm; GTV volume, 41.73 cm3). Adapted with permission from 46, copyright 2021 Elsevier.
The published data on the impact of FAPI PET/CT on radiotherapy are shown in Table 1. However, differing FAPI variants and thresholds for various types of tumors may be obstacles to the widespread use of FAPI PET/CT in GTV contouring. A well-designed prospective study with a large patient population is warranted to evaluate the overall survival benefit from FAPI-derived GTVs compared with GTVs derived from SCI in a heterogeneous grouping of cancers and imaging modalities.
Table 1.
Studies summarizing the impact of fibroblast activation protein inhibitor positron emission tomography/computed tomography (FAPI PET/CT) on the efficacy of radiotherapy.
| Study | Patients No. | Tumor type | FAPI variants | Compared imaging modalities | Background | FAPI thresholds | Results / optimal threshold |
|---|---|---|---|---|---|---|---|
| Windisch et al. 62 | 12 | Glioblastoma | 68Ga-FAPI-02 and 68Ga-FAPI-04 | CE-MRI | Healthy appearing contralateral brain parenchyma | FAPI × 5, 7, and 10 | FAP × 5, × 7 and × 10 increase the MRI-GTV (statistical significance |
| Syed et al. 60 | 14 | Head and neck cancers | 68Ga-FAPI | CE-CT and MRI | Healthy appearing surrounding tissue | FAPI × 3, 5, 7, and 10 | FAPI × 3 (about 20-25% SUVmax) |
| Röhrich et al. 63 | 12 | Adenoid cystic carcinomas | 68Ga-FAPI-02, 68Ga-FAPI-46, and 68Ga-FAPI-74 | CE-CT and CE-MRI | NA | 25-35% of SUVmax at three time points (10 min, 1 h, and 3 h) | The FAPI images acquired 1 h p.i. were considered ideal for contouring |
| Ristau et al. 64 | 7 | Esophageal cancer | 68Ga-FAPI-04 and 68Ga-FAPI-46 | Standard CT | Not mentioned | Not mentioned | FAPI PET/CT imaging improved target volume delineation in 6/7 patients |
| Zhao et al. 46 | 21 | Esophageal cancer | 68Ga-FAPI-04 | CE-CT | NA | FAPI × 20%, 30%, and 40% SUVmax | FAPI × 20% SUVmax |
| Giesel et al. 21 | 10 | Lung cancer | 18F-FAPI-74 and 68Ga-FAPI-74 | CE-CT | Blood-pool | FAPI × 1.5, 2, 2.5, and 3 | FAPI × 3 (about 40-50% SUVmax) |
| Koerber et al. 59 | 6 | Treatment-naïve carcinoma of the anal canal | 68Ga-FAPI-04 and 68Ga-FAPI-46 | MRI | Not mentioned | Not mentioned | Modified dose concepts in two patients, improved target volume delineation in six patients |
| Liermann et al. 61 | 7 | Locally recurrent pancreatic cancer | 68Ga-FAPI-04 | CE-CT | Healthy appearing surrounding tissue | FAPI × 1.5, 2, and 2.5 | FAPI × 2 |
CE: contrast-enhanced; CT: computed tomography; MRI: magnetic resonance imaging; NA: not applicable
Improvement in the FAPI probe and FAP-targeted radionuclide therapy
As a pan-cancer target with an excellent TBR, FAP is considered an attractive target for radionuclide therapy. FAPI variants labeled with therapeutic radionuclides (such as 131I, 90Y, 177Lu, and 225Ac) have been assessed in both preclinical and clinical studies. For example, Ma et al. synthesized 131I-FAPI-04 and used it to suppress tumor growth in U87MG glioma xenografts 65. FAPI-04 labeled with 225Ac demonstrated statistically significant tumor-suppressive effects compared with the control group in a study of pancreatic cancer xenografts 66. Similarly, 177Lu-FAPI-46 and 225Ac-FAPI-46 showed tumor growth suppression in pancreatic cancer mouse models without an obvious decrease in body weight; no radionuclide therapy-related side effects were observed in these tumor xenografts 67. However, tumor uptake was only 0.3% ID/g at 3 h p.i. and 0.1 % ID/g at 24 h p.i. for 177Lu-FAPI-46 and 225Ac-FAPI-46 67, respectively, and these results require additional clarification.
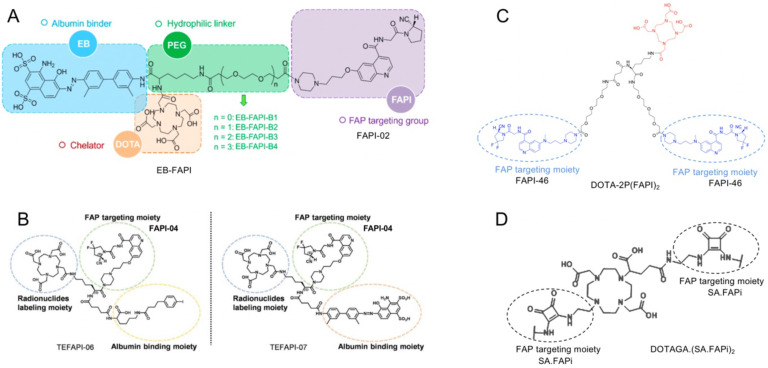
Strategies for prolonging the blood circulation of drug molecules by adding albumin-binder moieties and harnessing the polyvalency effects of multimeric peptides are widely used to enhance the tumor uptake and retention of radiopharmaceuticals 68, 69. For example, a series of albumin binder (truncated Evans blue) modified FAPI-02 related radiopharmaceuticals has been synthesized and radiolabeled with 177LuCl3 (named 177Lu-EB-FAPI-B1, B2, B3, B4, Figure 5A). Improved tumor accumulation and retention of these compounds were observed until 96 h post-injection, especially for 177Lu-EB-FAPI-B1. 177Lu-EB-FAPI-B1 demonstrated notable tumor growth inhibitions in the U87MG tumor model with negligible side effects, indicating that 177Lu-EB-FAPI-B1 is a promising theranostic agent for future clinical transformation 70. Similarly, FAPI-04 conjugated with albumin binder (4-[p-iodophenyl] butyric acid moiety, truncated Evans blue moiety, lauric acid [C12], and palmitic acid [C16]) was developed (TEFAPI-06, TEFAPI-07, FAPI-C12, and FAPI-C16) to improve tumor retention (Figure 5B), and novel FAPI-variants showed notable tumor growth inhibition after radiolabeling with 177Lu in pancreatic cancer patient-derived xenografts (PDXs) and HT-1080-FAP xenografts 71, 72. Another albumin binder (Lys [4-p-chlorophenyl] butyric acid)-conjugated FAP-targeting peptide (Alb-FAPtp-01) showed higher tumor uptake as compared with FAPI-04 after radiolabelling with 68Ga 73. Multimerization has been used as another strategy to improve tumor uptake and retention. Recently, the FAPI dimer DOTA-2P(FAPI)2 was synthesized based on the structure of FAPI-46 (Figure 5C), and it demonstrated increased tumor uptake and retention properties compared to FAPI-46 in PDXs of hepatocellular carcinoma 74. Moreover, PET/CT scans in three cancer patients revealed higher intratumoral uptake of 68Ga-DOTA-2P(FAPI)2 compared to 68Ga-FAPI-46 in 21 tumor lesions (SUVmax: 8.1-39.0 vs. 1.7-24.0; P < 0.001) 74.
Figure 5.
A. Chemical structure and each part of the functional groups of 177Lu-EB-FAPI-B1 (without PEG), 177Lu-EB-FAPI-B2 (with PEG: n = 1), 177Lu-EB-FAPI-B3 (with PEG: n = 2) and 177Lu-EB-FAPI-B4 (with PEG: n = 3) (FAP targeting motif: FAPI-02). Adapted with permission from 70, copyright 2021 Ivyspring. B-D. (B) Chemical structure of TEFAPI-06/07 (FAP targeting motif: FAPI-04). Adapted with permission from 71, copyright 2021 Journal of Nuclear Medicine. (C) Chemical structure of DOTA-2P(FAPI)2 (FAP targeting motif: FAPI-46). Adapted with permission from 74, copyright 2021 Journal of Nuclear Medicine. (D) Chemical structure of DOTAGA.(SA.FAPi)2 (FAP targeting motif: SA.FAPi) Adapted with permission from 83, copyright 2021 Mary Ann Liebert.
Regarding the clinical investigation of FAPI-targeted radionuclide therapy, Lindner et al. first reported that a patient with advanced breast cancer was treated with 2.9 GBq of 90Y-FAPI-04, resulting in a statistically significant reduction in pain medication 17. Other FAPI variants (FAPI-46 and DOTA.SA.FAPi) radiolabeled with therapeutic nuclides (153Sm, 90Y, and 177Lu) were evaluated in several scattered case reports (Table 2) 75, 22, 76-78. Subsequently, a few preliminary studies on FAP-targeted radionuclide treatment were reported.
Table 2.
Radionuclide therapy targeting fibroblast activation protein (FAP).
| Study | Patients No. | Tumor type | FAPI agent | Total cycles | Treatment cycle/ patient |
Median injected activity | Response (RECIST) | Treatment-related adverse events in all treatment cycles |
|---|---|---|---|---|---|---|---|---|
| Assadi et al. 81 | 18 | Ovarian cancer, sarcoma, colon cancer, breast cancer, pancreatic cancer, prostate cancer, cervical cancer, round-cell tumor, lung cancer, anaplastic thyroid cancer, cholangiocarcinoma | 177Lu-FAPI-46 | 36 | 1-4 | 3.7 GBq (1.85-13.7 GBq) | 12 SD, 6 PD | 1 patient suffered thrombocytopenia (G1), leukopenia (G1), and anaemia (G3) (CTCAE v4.03) |
| Ballal et al. 83 | 15 | Thyroid Cancer | 177Lu-DOTAGA.(SA.FAPi)2 | 45 | 2-4 | 8.2 GBq (5.5-14 GBq) | NA | Diarrhoea (G1 in 1 pt) (CTCAE v5.0) |
| Baum et al. 79 | 11 | Pancreatic cancer, breast cancer, ovarian cancer, and rectum cancer | 177Lu-FAP-2286 | 22 | 1-3 | 5.8 GBq (2.4-9.9 GBq)a | 2 SD, 9 PD | Hemoglobin (G1 in 2 pts, G2 in 4 pts, and G3 in 1 pt), leukopenia (G2 in 1 pt, and G3 in 2 pts, non-G3 in 2 pts), thrombocytopenia (G3 in 1 pts),b pain flare-up (G3 in 1 pts) (CTCAE v5.0) |
| Ferdinandus et al. 80 | 9 | Sarcoma, pancreatic cancer | 90Y-FAPI-46 | 13 | 1-3 | 3.8 (3.25-5.40) GBq for the first cycle and 7.4 (7.3-7.5) GBq for any subsequent cycle | 4 SD, 4 PD | Hemoglobin (G1 in 2 pts, G2 in 2 pts, and G3 in 4 pts), kidney adverse events (G1 in 1 pt, and G2 in 2 pts), liver adverse events (G1 in 1 pt, G2 in 2 pts, G3 in 1 pt, and G4 in 1 pt), pancreatobiliary adverse events (G1 in 1 pt, G3 in 1 pt, and G4 in 1 pt) (CTCAE v5.0) |
| Kuyumcu et al. 82 | 4 | Breast cancer, thymic carcinoma, thyroid cancer, ovarian carcinosarcoma | 177Lu-FAPI-04 | 4 | 1 | 0.27 GBq (0.26-0.28 GBq) | NA | NA |
| Lindner et al. 22 | 2 | Ovarian cancer and pancreatic cancer | 90Y‐FAPI‐46 | 2 | 1 | 6 GBq | NA | NA |
| Jokar et al. 78 | 1 | Breast cancer | 177Lu-FAPI-46 | 2 | 2 | 3.7 GBq | NA | NA |
| Rathke et al. 77 | 1 | Metachronous metastasized breast cancer and colorectal cancer | 90Y-FAPI-46 | 4 | 4 | 35.5 GBq | SD for breast cancer and PR for colorectal cancer after 1 cycle, but PD after 4 cycles | NA |
| Ballal S et al. 76 | 1 | Breast cancer | 177Lu-DOTA.SA.FAPi | 1 | 1 | 3.2 GBq | Decrease in the intensity of headaches | NA |
| Lindner et al. 17 | 1 | Breast cancer | 90Y-FAPI-04 | 1 | 1 | 2.9 GBq | Statistically significant reduction in pain medication | NA |
| Kratochwil et al. 75 | 1 | Sarcoma | 153Sm-FAPI-46 90Y-FAPI-46 | 3 | 3 | 20 GBq for 153Sm and 8 GBq for 90Y | SD | NA |
FAPI: fibroblast activation protein inhibitor; RECIST: Response Evaluation Criteria in Solid Tumors; SD: stable disease; PD: progressive disease; PR: partial response; NA: not applicable; CTCAE: Common Terminology Criteria for Adverse Events.
aThis footnote indicates the presentation of means rather than medians.
bOne patient with hemoglobin (G3), leukopenia (G3), and thrombocytopenia (G3).
For example, in a study of 22 cycles of 177Lu-FAP-2286 administered to 11 patients with diverse adenocarcinomas (mean injected activity, 5.8 GBq), grade 3 adverse events occurred in three patients, and no grade 4/5 adverse events occurred 79. Ferdinandus et al. reported 13 cycles of 90Y-FAPI-46 administered to nine patients (with a mean injected activity of 3.8 GBq for the first cycle and a mean injected activity of 7.4 GBq for any subsequent cycle), with new grade 3/4 adverse events occurring in four patients 80. In contrast, only one patient had a new grade 3 adverse event in a study of 36 cycles of 177Lu-FAPI-46 administered to 18 patients (median injected activity, 3.7 GBq) 81. The measured mean absorbed dose of 177Lu-FAPI-04 was 0.37 Gy/GBq in tumor lesions 82, much lower than that of 90Y-FAPI-46 (median, 1.28 Gy/GBq) and 177Lu-FAP-2286 (3.00 Gy/GBq in bone metastases) 80, 79. Recently, another FAPI dimer, DOTA(SA.FAPi)2, was developed and synthesized by Ballal et al. (Figure 5D) 83. Radionuclide therapy with 177Lu-DOTA(SA.FAPi)2 was administered to 15 patients with radioiodine-refractory differentiated thyroid cancer (DTC; RR-DTC). The results of that study revealed that the numbers of patients with complete response, partial response, and stable disease were 0, 4, and 3, respectively. None of the patients experienced grade 3/4 hematological, renal, or hepato-toxicity.
However, most of the studies mentioned above showed mixed responses to FAP-targeted radionuclide therapy because of the different tumors and patient conditions evaluated (Table 2). It should be noted that the latest studies were mainly aimed at evaluating the feasibility and safety of FAP-targeted radionuclide therapy; the number of patients enrolled in these studies was very limited, and the patient cohorts were heterogeneous. In addition, most patients received FAP-targeted radionuclide therapy as the last line of treatment with poor performance status. Although the tumor half-life of FAP-2286 (average of 44 h for bone and 32 h for single liver metastases) is prolonged compared to FAPI-02/04, it is still shorter than the tumor half-life of PSMA 79, 84.
PSMA-targeted radionuclide therapy is reportedly very effective with beta-radionuclides such as 177Lu. Moreover, 177Lu-PSMA directly targets cancer cells in radiotherapy, while the ionizing radiation of radiolabeled FAPIs mainly kills CAFs and indirectly kills cancer cells adjacent to CAFs via crossfire effects. These reasons may partially explain the difference in treatment response to targeted radionuclide therapy between PSMA and FAPI. Therefore, further research to enhance the therapeutic efficacy of FAP-targeted radionuclide is of great importance, including optimizing the chemical structure of the FAPI vector (e.g., multimerization and chemical conjugation with albumin binder), shortening the time interval between treatments, increasing the administered dose of therapeutic radionuclide, and combination treatments with other types of treatment (e.g., immunotherapy, external-beam radiotherapy, and molecular targeted therapy).
It has been reported that a tumor size of 1-2 mm requires the formation of stroma to support the tumor 57. Thus, radionuclide therapy targeting FAP may be highly effective for treating advanced cancer patients with widespread metastases. Moreover, in an autochthonous model of pancreatic ductal adenocarcinoma, depleting FAP-positive CAFs induced T-cell accumulation in cancer cells and synergistically enhanced anti-tumor effects within PD-L1 immunotherapy 85. Therefore, exploring optimal combination therapies with radionuclide therapy targeting FAP, especially with respect to immunotherapy, is warranted in future research. It must be noted that FAP is overexpressed in various epithelial cancers and is also expressed in many non-oncological diseases 23. In a cohort of 91 patients, 81.3% of the presenting patients had non-tumor FAPI uptake, including degenerative lesions and physiological uptake in normal salivary glands, mammary glands, and the uterus 86. In order to select patients who are most likely to benefit from this therapeutic regimen, careful pre-therapeutic evaluation with 68Ga-FAPI PET/CT is recommended prior to FAP-targeted radionuclide therapy.
Conclusion
FAPI variants labeled with 68Ga or 18F have shown impressive results in a broad spectrum of cancers. Well-designed clinical trials with large patient populations are needed to define the role of this diagnostic agent, as 18F-FDG is the dominant tracer in clinical oncology at present. In addition to CAFs, intense FAP expression is also related to fibrosis, arthritis, atherosclerosis, and autoimmune diseases. Thus, FAPI uptake in non-malignant diseases must be carefully identified. Regarding FAP-targeted radionuclide therapy, one direction for future research is improving the pharmacokinetic properties of tracers via chemical modification. The other potential direction is to explore optimal combination therapies (e.g., combining with external-beam radiotherapy, chemotherapy, and immunotherapy) to synergistically enhance anti-tumor efficacy. Overall, FAPI-based imaging and therapy of cancer have been a highly vibrant research field over the past few years. We look forward to future studies and rapid translation of the most promising FAPI ligands into the clinical arena to benefit patients with various types of cancer.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Grant number 82071961 and 81772893) and Key Medical and Health Projects in Xiamen (Grant number 3502Z20209002).
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose
- CAFs
cancer-associated fibroblasts
- CCL2
CC-chemokine ligand 2
- CE-CT
contrast-enhanced computed tomography
- CE-MRI
contrast-enhanced magnetic resonance imaging
- CUP
cancers of unknown primary origin
- DOTA
dodecane tetraacetic acid
- ECM
extracellular matrix
- FAP
fibroblast activation protein
- FAPIs
FAP inhibitors
- GGO
ground-glass opacity
- GTV
gross tumor volume
- IL-6
interleukin-6
- OSCC
oral squamous cell carcinoma
- PDGFRβ
platelet-derived growth factor receptor-β
- PDXs
patient-derived xenografts
- PET
positron emission tomography
- p.i.
post injection
- SCI
standard care of imaging
- TBR
target-to-blood pool ratio
- TGFβ
transforming growth factor-β
- TME
tumor microenvironment
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 3.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 5.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W. et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016;76(14):4124–35. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 7.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–37. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg TP, Stuart M, Oosting J, Tollenaar R, Sier CFM, Mesker WE. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer. 2019;19(1):284. doi: 10.1186/s12885-019-5462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Ruiz P, Corvigno S, Te Grootenhuis NC, La Fleur L, Backman M, Strell C. et al. Stromal FAP is an independent poor prognosis marker in non-small cell lung adenocarcinoma and associated with p53 mutation. Lung Cancer. 2021;155:10–9. doi: 10.1016/j.lungcan.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Zou B, Liu X, Zhang B, Gong Y, Cai C, Li P. et al. The Expression of FAP in Hepatocellular Carcinoma Cells is Induced by Hypoxia and Correlates with Poor Clinical Outcomes. J Cancer. 2018;9(18):3278–86. doi: 10.7150/jca.25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikberg ML, Edin S, Lundberg IV, Van Guelpen B, Dahlin AM, Rutegard J. et al. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumour Biol. 2013;34(2):1013–20. doi: 10.1007/s13277-012-0638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng JD, Dunbrack RL Jr, Valianou M, Rogatko A, Alpaugh RK, Weiner LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002;62(16):4767–72. [PubMed] [Google Scholar]
- 14.Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jager D, Renner C. et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26(1):44–8. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39(3):783–803. doi: 10.1007/s10555-020-09909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jager D. et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J Nucl Med. 2018;59(9):1423–9. doi: 10.2967/jnumed.118.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J. et al. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med. 2018;59(9):1415–22. doi: 10.2967/jnumed.118.210443. [DOI] [PubMed] [Google Scholar]
- 18.Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C. et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J Nucl Med. 2019;60(10):1421–9. doi: 10.2967/jnumed.118.224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W. et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med. 2019;60(6):801–5. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferdinandus J, Kessler L, Hirmas N, Trajkovic-Arsic M, Hamacher R, Umutlu L. et al. Equivalent tumor detection for early and late FAPI-46 PET acquisition. Eur J Nucl Med Mol Imaging. 2021;48(10):3221–7. doi: 10.1007/s00259-021-05266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giesel FL, Adeberg S, Syed M, Lindner T, Jimenez-Franco LD, Mavriopoulou E. et al. FAPI-74 PET/CT Using Either (18)F-AlF or Cold-Kit (68)Ga Labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer Patients. J Nucl Med. 2021;62(2):201–7. doi: 10.2967/jnumed.120.245084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindner T, Altmann A, Kramer S, Kleist C, Loktev A, Kratochwil C. et al. Design and Development of (99m)Tc-Labeled FAPI Tracers for SPECT Imaging and (188)Re Therapy. J Nucl Med. 2020;61(10):1507–13. doi: 10.2967/jnumed.119.239731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng S, Lin R, Chen S, Zheng J, Lin Z, Zhang Y. et al. Characterization of the benign lesions with increased (68)Ga-FAPI-04 uptake in PET/CT. Ann Nucl Med. 2021;35(12):1312–1320. doi: 10.1007/s12149-021-01673-w. [DOI] [PubMed] [Google Scholar]
- 24.Qin C, Song Y, Liu X, Gai Y, Liu Q, Ruan W, Increased uptake of (68)Ga-DOTA-FAPI-04 in bones and joints: metastases and beyond. Eur J Nucl Med Mol Imaging. 2021. doi: 10.1007/s00259-021-05472-3. [DOI] [PubMed]
- 25.Schmidkonz C, Rauber S, Atzinger A, Agarwal R, Gotz TI, Soare A. et al. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann Rheum Dis. 2020;79(11):1485–91. doi: 10.1136/annrheumdis-2020-217408. [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Ning J, Li J, Lai Z, Shi X, Xing H. et al. Feasibility of in vivo Imaging of Fibroblast Activation Protein in Human Arterial Walls. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262863. doi: 10.2967/jnumed.121.262863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohrich M, Leitz D, Glatting FM, Wefers AK, Weinheimer O, Flechsig P. et al. Fibroblast Activation Protein specific PET/CT imaging in fibrotic interstitial lung diseases and lung cancer: a translational exploratory study. J Nucl Med. 2021 doi: 10.2967/jnumed.121.261925. doi: 10.2967/jnumed.121.261925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergmann C, Distler J, Treutlein C, Tascilar K, Schmidkonz CJTLR. 68Ga-FAPI-04 PET-CT for molecular assessment of fibroblast activation and risk evaluation in systemic sclerosis-associated interstitial lung disease: a single-centre, pilot study. Lancet Rheumatol. 2021;3:e185–94. doi: 10.1016/S2665-9913(20)30421-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J. et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47(8):1820–32. doi: 10.1007/s00259-020-04769-z. [DOI] [PubMed] [Google Scholar]
- 30.Gu B, Xu X, Zhang J, Ou X, Xia Z, Guan Q. et al. The Added Value of (68)Ga-FAPI-04 PET/CT in Patients with Head and Neck Cancer of Unknown Primary with (18)F-FDG Negative Findings. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262790. doi:10.2967/jnumed.121.262790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Pang Y, Zheng H, Han C, Gu J, Sun L. et al. Clinical utility of [(68)Ga]Ga-labeled fibroblast activation protein inhibitor (FAPI) positron emission tomography/computed tomography for primary staging and recurrence detection in nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging. 2021;48(11):3606–17. doi: 10.1007/s00259-021-05336-w. [DOI] [PubMed] [Google Scholar]
- 32.Qin C, Liu F, Huang J, Ruan W, Liu Q, Gai Y. et al. A head-to-head comparison of (68)Ga-DOTA-FAPI-04 and (18)F-FDG PET/MR in patients with nasopharyngeal carcinoma: a prospective study. Eur J Nucl Med Mol Imaging. 2021;48(10):3228–37. doi: 10.1007/s00259-021-05255-w. [DOI] [PubMed] [Google Scholar]
- 33.Linz C, Brands RC, Kertels O, Dierks A, Brumberg J, Gerhard-Hartmann E. et al. Targeting fibroblast activation protein in newly diagnosed squamous cell carcinoma of the oral cavity - initial experience and comparison to [(18)F]FDG PET/CT and MRI. Eur J Nucl Med Mol Imaging. 2021;48(12):3951–60. doi: 10.1007/s00259-021-05422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serfling S, Zhi Y, Schirbel A, Lindner T, Meyer T, Gerhard-Hartmann E. et al. Improved cancer detection in Waldeyer's tonsillar ring by (68)Ga-FAPI PET/CT imaging. Eur J Nucl Med Mol Imaging. 2021;48(4):1178–87. doi: 10.1007/s00259-020-05055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shang Q, Zhao L, Pang Y, Yu Y, Chen H. 68Ga-FAPI PET/CT Distinguishes the Reactive Lymph Nodes From Tumor Metastatic Lymph Nodes in a Patient With Nasopharyngeal Carcinoma. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003939. doi:10.1097/RLU.0000000000003939. [DOI] [PubMed] [Google Scholar]
- 36.Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J. et al. Imaging fibroblast activation protein in liver cancer: a single-center post hoc retrospective analysis to compare [(68)Ga]Ga-FAPI-04 PET/CT versus MRI and [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(5):1604–17. doi: 10.1007/s00259-020-05095-0. [DOI] [PubMed] [Google Scholar]
- 37.Shi X, Xing H, Yang X, Li F, Yao S, Congwei J. et al. Comparison of PET imaging of activated fibroblasts and (18)F-FDG for diagnosis of primary hepatic tumours: a prospective pilot study. Eur J Nucl Med Mol Imaging. 2021;48(5):1593–603. doi: 10.1007/s00259-020-05070-9. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J. et al. (68)Ga-FAPI-04 Versus (18)F-FDG PET/CT in the Detection of Hepatocellular Carcinoma. Front Oncol. 2021;11:693640. doi: 10.3389/fonc.2021.693640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q. et al. Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology. 2021;298(2):393–402. doi: 10.1148/radiol.2020203275. [DOI] [PubMed] [Google Scholar]
- 40.Qin C, Shao F, Gai Y, Liu Q, Ruan W, Liu F. et al. (68)Ga-DOTA-FAPI-04 PET/MR in the evaluation of gastric carcinomas: comparison with (18)F-FDG PET/CT. J Nucl Med. 2021 doi: 10.2967/jnumed.120.258467. doi:10.2967/jnumed.120.258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang D, Chen X, You Z, Wang H, Zhang X, Li X, Comparison of [(68) Ga]Ga-FAPI-04 and [(18)F]-FDG for the detection of primary and metastatic lesions in patients with gastric cancer: a bicentric retrospective study. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05441-w. [DOI] [PubMed]
- 42.Kuten J, Levine C, Shamni O, Pelles S, Wolf I, Lahat G, Head-to-head comparison of [(68)Ga]Ga-FAPI-04 and [(18)F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05494-x. [DOI] [PMC free article] [PubMed]
- 43.Pang Y, Zhao L, Shang Q, Meng T, Zhao L, Feng L, Positron emission tomography and computed tomography with [(68)Ga]Ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05576-w. [DOI] [PubMed]
- 44.Rohrich M, Naumann P, Giesel FL, Choyke PL, Staudinger F, Wefers A. et al. Impact of (68)Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J Nucl Med. 2021;62(6):779–86. doi: 10.2967/jnumed.120.253062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gundogan C, Komek H, Can C, Yildirim OA, Kaplan I, Erdur E. et al. Comparison of 18F-FDG PET/CT and 68Ga-FAPI-04 PET/CT in the staging and restaging of gastric adenocarcinoma. Nucl Med Commun. 2021 doi: 10.1097/MNM.0000000000001489. doi:10.1097/MNM.0000000000001489. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L, Chen S, Chen S, Pang Y, Dai Y, Hu S. et al. (68)Ga-fibroblast activation protein inhibitor PET/CT on gross tumour volume delineation for radiotherapy planning of oesophageal cancer. Radiother Oncol. 2021;158:55–61. doi: 10.1016/j.radonc.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Elboga U, Sahin E, Kus T, Cayirli YB, Aktas G, Uzun E, Superiority of (68)Ga-FAPI PET/CT scan in detecting additional lesions compared to (18)FDG PET/CT scan in breast cancer. Ann Nucl Med. 2021. doi:10.1007/s12149-021-01672-x. [DOI] [PubMed]
- 48.Komek H, Can C, Guzel Y, Oruc Z, Gundogan C, Yildirim OA. et al. (68)Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: a comparative pilot study with the (18)F-FDG PET/CT. Ann Nucl Med. 2021;35(6):744–52. doi: 10.1007/s12149-021-01616-5. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Lin X, Li Y, Lv J, Hou P, Liu S, Clinical Utility of F-18 Labeled Fibroblast Activation Protein Inhibitor (FAPI) for Primary Staging in Lung Adenocarcinoma: a Prospective Study. Mol Imaging Biol. 2021. doi:10.1007/s11307-021-01679-w. [DOI] [PubMed]
- 50.Chen H, Pang Y, Meng T, Yu X, Sun L. 18F-FDG and 68Ga-FAPI PET/CT in the Evaluation of Ground-Glass Opacity Nodule. Clin Nucl Med. 2021;46(5):424–6. doi: 10.1097/RLU.0000000000003600. [DOI] [PubMed] [Google Scholar]
- 51.Kessler L, Ferdinandus J, Hirmas N, Bauer S, Dirksen U, Zarrad F. et al. Ga-68-FAPI as diagnostic tool in sarcoma: Data from the FAPI-PET prospective observational trial. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262096. doi:10.2967/jnumed.121.262096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan L, Liu H, Wang Y, Deng J, Peng D, Feng Y, The potential utility of [(68) Ga]Ga-DOTA-FAPI-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [(18)F]FDG. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05522-w. [DOI] [PubMed]
- 53.Treglia G, Muoio B, Roustaei H, Kiamanesh Z, Aryana K, Sadeghi R. Head-to-Head Comparison of Fibroblast Activation Protein Inhibitors (FAPI) Radiotracers versus [(18)F]F-FDG in Oncology: A Systematic Review. Int J Mol Sci. 2021. 22(20). doi:10.3390/ijms222011192. [DOI] [PMC free article] [PubMed]
- 54.Sollini M, Kirienko M, Gelardi F, Fiz F, Gozzi N, Chiti A. State-of-the-art of FAPI-PET imaging: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05475-0. [DOI] [PubMed]
- 55.Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y. et al. Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. 2021;48(1):73–86. doi: 10.1007/s00259-020-04940-6. [DOI] [PubMed] [Google Scholar]
- 56.Giesel FL, Kratochwil C, Schlittenhardt J, Dendl K, Eiber M, Staudinger F, Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05307-1. [DOI] [PMC free article] [PubMed]
- 57.Calais J. FAP: The Next Billion Dollar Nuclear Theranostics Target? J Nucl Med. 2020;61(2):163–5. doi: 10.2967/jnumed.119.241232. [DOI] [PubMed] [Google Scholar]
- 58.Zhao L, Chen S, Lin L, Sun L, Wu H, Lin Q. et al. [(68)Ga]Ga-DOTA-FAPI-04 improves tumor staging and monitors early response to chemoradiotherapy in a patient with esophageal cancer. Eur J Nucl Med Mol Imaging. 2020;47(13):3188–9. doi: 10.1007/s00259-020-04818-7. [DOI] [PubMed] [Google Scholar]
- 59.Koerber SA, Staudinger F, Kratochwil C, Adeberg S, Haefner MF, Ungerechts G. et al. The role of FAPI-PET/CT for patients with malignancies of the lower gastrointestinal tract - first clinical experience. J Nucl Med. 2020 doi: 10.2967/jnumed.119.237016. doi:10.2967/jnumed.119.237016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syed M, Flechsig P, Liermann J, Windisch P, Staudinger F, Akbaba S. et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur J Nucl Med Mol Imaging. 2020;47(12):2836–45. doi: 10.1007/s00259-020-04859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liermann J, Syed M, Ben-Josef E, Schubert K, Schlampp I, Sprengel SD. et al. Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer. Cancers (Basel) 2021;13(4):796. doi: 10.3390/cancers13040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Windisch P, Rohrich M, Regnery S, Tonndorf-Martini E, Held T, Lang K. et al. Fibroblast Activation Protein (FAP) specific PET for advanced target volume delineation in glioblastoma. Radiother Oncol. 2020;150:159–63. doi: 10.1016/j.radonc.2020.06.040. [DOI] [PubMed] [Google Scholar]
- 63.Rohrich M, Syed M, Liew DP, Giesel FL, Liermann J, Choyke PL. et al. (68)Ga-FAPI-PET/CT improves diagnostic staging and radiotherapy planning of adenoid cystic carcinomas - Imaging analysis and histological validation. Radiother Oncol. 2021;160:192–201. doi: 10.1016/j.radonc.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ristau J, Giesel FL, Haefner MF, Staudinger F, Lindner T, Merkel A. et al. Impact of Primary Staging with Fibroblast Activation Protein Specific Enzyme Inhibitor (FAPI)-PET/CT on Radio-Oncologic Treatment Planning of Patients with Esophageal Cancer. Mol Imaging Biol. 2020;22(6):1495–500. doi: 10.1007/s11307-020-01548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma H, Li F, Shen G, Cai H, Liu W, Lan T. et al. Synthesis and Preliminary Evaluation of (131)I-Labeled FAPI Tracers for Cancer Theranostics. Mol Pharm. 2021;18(11):4179–87. doi: 10.1021/acs.molpharmaceut.1c00566. [DOI] [PubMed] [Google Scholar]
- 66.Watabe T, Liu Y, Kaneda-Nakashima K, Shirakami Y, Lindner T, Ooe K. et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: (64)Cu- and (225)Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J Nucl Med. 2020;61(4):563–9. doi: 10.2967/jnumed.119.233122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Watabe T, Kaneda-Nakashima K, Shirakami Y, Naka S, Ooe K, Fibroblast activation protein targeted therapy using [(177)Lu]FAPI-46 compared with [(225)Ac]FAPI-46 in a pancreatic cancer model. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05554-2. [DOI] [PMC free article] [PubMed]
- 68.Li ZB, Cai W, Cao Q, Chen K, Wu Z, He L. et al. (64)Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor alpha(v)beta(3) integrin expression. J Nucl Med. 2007;48(7):1162–71. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z, Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45(5):1432–56. doi: 10.1039/c5cs00158g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen X, Xu P, Shi M, Liu J, Zeng X, Zhang Y. et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics. 2022;12(1):422–33. doi: 10.7150/thno.68182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu M, Zhang P, Ding J, Chen J, Huo L, Liu Z. Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262533. doi:10.2967/jnumed.121.262533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang P, Xu M, Ding J, Chen J, Zhang T, Huo L, Fatty acid-conjugated radiopharmaceuticals for fibroblast activation protein-targeted radiotherapy. Eur J Nucl Med Mol Imaging. 2021. doi:10.1007/s00259-021-05591-x. [DOI] [PubMed]
- 73.Lin JJ, Chuang CP, Lin JY, Huang FT, Huang CW. Rational Design, Pharmacomodulation, and Synthesis of [(68)Ga]Ga-Alb-FAPtp-01, a Selective Tumor-Associated Fibroblast Activation Protein Tracer for PET Imaging of Glioma. ACS Sens. 2021;6(9):3424–35. doi: 10.1021/acssensors.1c01316. [DOI] [PubMed] [Google Scholar]
- 74.Zhao L, Niu B, Fang J, Pang Y, Li S, Xie C. et al. Synthesis, preclinical evaluation, and a pilot clinical PET imaging study of (68)Ga-labeled FAPI dimer. J Nucl Med. 2021 doi: 10.2967/jnumed.121.263016. doi:10.2967/jnumed.121.263016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kratochwil C, Giesel FL, Rathke H, Fink R, Dendl K, Debus J. et al. [(153)Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur J Nucl Med Mol Imaging. 2021;48(9):3011–3. doi: 10.1007/s00259-021-05273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballal S, Yadav MP, Kramer V, Moon ES, Roesch F, Tripathi M. et al. A theranostic approach of [(68)Ga]Ga-DOTA.SA.FAPi PET/CT-guided [(177)Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: new frontier in targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. 2021;48(3):942–4. doi: 10.1007/s00259-020-04990-w. [DOI] [PubMed] [Google Scholar]
- 77.Rathke H, Fuxius S, Giesel FL, Lindner T, Debus J, Haberkorn U. et al. Two Tumors, One Target: Preliminary Experience With 90Y-FAPI Therapy in a Patient With Metastasized Breast and Colorectal Cancer. Clin Nucl Med. 2021;46(10):842–4. doi: 10.1097/RLU.0000000000003842. [DOI] [PubMed] [Google Scholar]
- 78.Jokar N, Velikyan I, Ahmadzadehfar H, Rekabpour SJ, Jafari E, Ting HH. et al. Theranostic Approach in Breast Cancer: A Treasured Tailor for Future Oncology. Clin Nucl Med. 2021;46(8):e410–e20. doi: 10.1097/RLU.0000000000003678. [DOI] [PubMed] [Google Scholar]
- 79.Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, Zhang J. et al. Feasibility, Biodistribution and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy (PTRT) of Diverse Adenocarcinomas using (177)Lu-FAP-2286: First-in-Human Results. J Nucl Med. 2021 doi: 10.2967/jnumed.120.259192. doi:10.2967/jnumed.120.259192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferdinandus J, Fragoso Costa P, Kessler L, Weber M, Hirmas N, Kostbade K. et al. Initial clinical experience with (90)Y-FAPI-46 radioligand therapy for advanced stage solid tumors: a case series of nine patients. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262468. doi:10.2967/jnumed.121.262468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Assadi M, Rekabpour SJ, Jafari E, Divband G, Nikkholgh B, Amini H. et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin Nucl Med. 2021;46(11):e523–e30. doi: 10.1097/RLU.0000000000003810. [DOI] [PubMed] [Google Scholar]
- 82.Kuyumcu S, Kovan B, Sanli Y, Buyukkaya F, Has Simsek D, Ozkan ZG. et al. Safety of Fibroblast Activation Protein-Targeted Radionuclide Therapy by a Low-Dose Dosimetric Approach Using 177Lu-FAPI04. Clin Nucl Med. 2021;46(8):641–6. doi: 10.1097/RLU.0000000000003667. [DOI] [PubMed] [Google Scholar]
- 83.Ballal S, Yadav MP, Moon ES, Roesch F, Kumari S, Agarwal S. et al. Novel Fibroblast Activation Protein Inhibitor-Based targeted Theranostics for Radioiodine Refractory differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid. 2021 doi: 10.1089/thy.2021.0412. doi:10.1089/thy.2021.0412. [DOI] [PubMed] [Google Scholar]
- 84.Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y. et al. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J Nucl Med. 2016;57(Suppl 3):97S–104S. doi: 10.2967/jnumed.115.170167. [DOI] [PubMed] [Google Scholar]
- 85.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(50):20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kessler L, Ferdinandus J, Hirmas N, Zarrad F, Nader M, Kersting D. et al. Pitfalls and common findings in (68)Ga-FAPI-PET - A pictorial analysis. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262808. doi:10.2967/jnumed.121.262808. [DOI] [PMC free article] [PubMed] [Google Scholar]