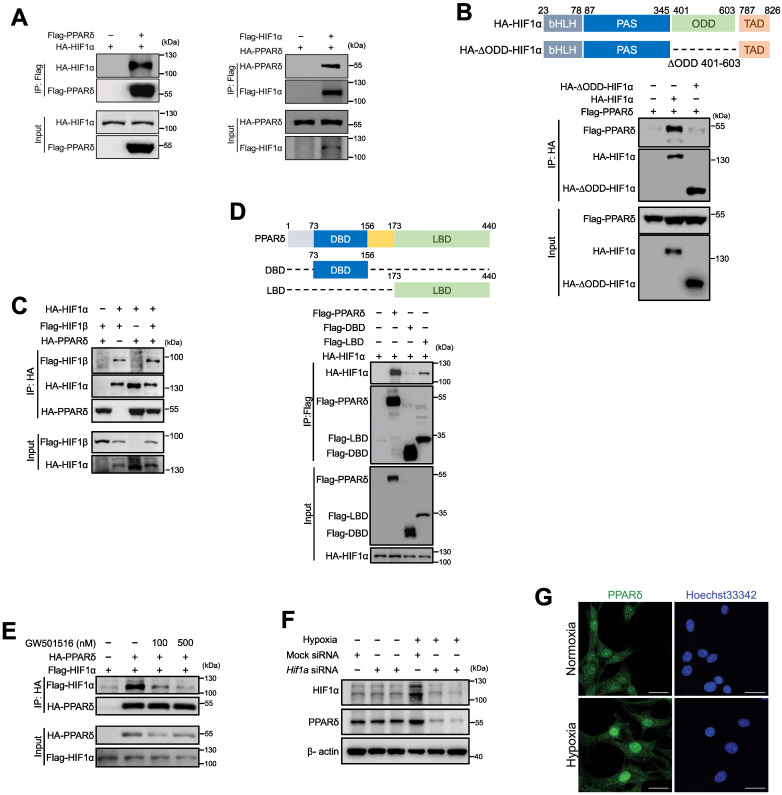
Figure 6.
Hypoxia-induced PPARδ interacts with HIF1α in endothelial cells. A, Immunoblots showing HIF1α and PPARδ in anti-Flag immunoprecipitates in HEK293T transfected with indicated plasmids. B, Schematic diagram showing site of ∆ODD in HIF1α gene and immunoblots showing the Flag-PPARδ in the anti-HA immunoprecipitates from cells co-expressing Flag-PPARδ and full-length HA-HIF1α or Flag-PPARδ and ODD domain deleted HIF1α (HA-∆ODD-HIF1α). C, Immunoblots showing the anti-HA immunoprecipitates in HEK293T with transfection of indicated plasmids. D, Schematic diagram showing the position of full-length and truncated PPARδ DBD and LBD, and immunoblots showing the anti-Flag immunoprecipitates in HEK293T with indicated plasmids transfected. E, Immunoblots showing the anti-HA or anti-Flag immunoprecipitates in HEK293T with indicated plasmids transfected to show the interaction of PPARδ and HIF1α treated with GW501516 (6 h) or solvent control. F, Immunoblots of protein expression after transfection with Hif1a siRNA in mBMECs after hypoxia for 12 h. G, Representative immunofluorescence of PPARδ localization in the nuclei of mBMECs after hypoxia for 12 h (n = 4 biological replicates of each group). Scale bar, 20 μm. All the siRNA transfections were performed with lipofectamine RNA iMax for 48 h, and all the plasmids were transfected with lipofectamine for 36 h, before other treatments. Representative data have at least three biological replicates (A-F).