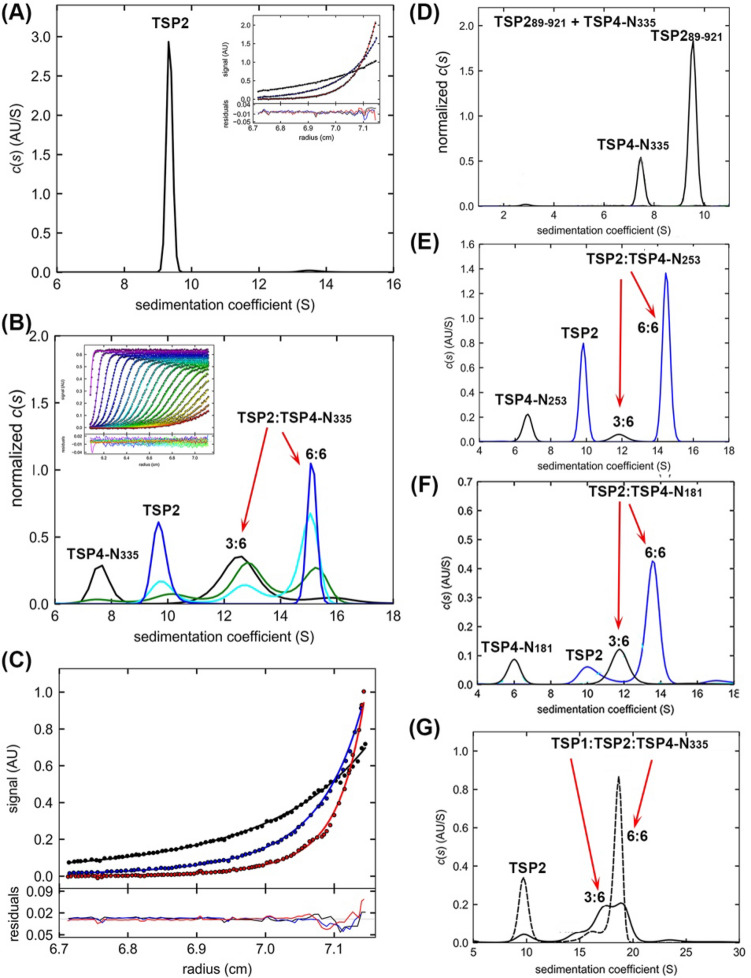
Figure 7.
AUC analyses of the association between TSP2 and TSP4-N proteins in the absence and presence of TSP1. (A) The c(s) distribution of 3.5 μM TSP2. Insert: SE profile of 3 μM TSP2 with a best fit RMSD of 0.01 AU, collected at 4, 6 and 8 krpm rotor speeds. The best fits are shown as black solid lines through the experimental data with MWapp of 270 kDa. (B) The SV profiles of 3.3 μM TSP4-N335 in the presence of different TSP2 concentrations: 1.7 μM (black), 3.3 μM (green), 5 μM (cyan), and 8 μM TSP2 (blue). Insert: Direct LEq modeling of SV data from 3.3 μM each of TSP2 and TSP4-N335 hexamer. The best fits are shown as solid lines through the experimental data. (C) SE profile of 1.3 μM TSP4-N355 and 1.3 μM TSP2 with a best fit RMSD of 0.012 AU, collected at 4, 6 and 8 krpm rotor speeds. The best fits are shown as solid lines through the experimental data. The LEq analysis of SV and SE data calculated with the program SEDPHAT are described in Fig. 6C. (D) The c(s) distribution profiles of 3 μM TSP4-N335 with 2 μM TSP289-921 lacking the XD2 domain. (E) The c(s) distribution profiles of 3 μM TSP4-N253 in the presence of 0.37 μM (black) and 2.5 μM TSP2 (blue). (F) The SV profiles of 3.16 μM TSP4-N181 in the presence of 0.37 μM (black) and 1.5 μM TSP2 (blue). (G) The c(s) distribution profiles of 3.3 μM each of TSP4-N335, TSP1 and TSP2 (solid line), and 3.3 μM each of TSP4-N335 and TSP1 with 6.6 μM TSP2 (dashed line).