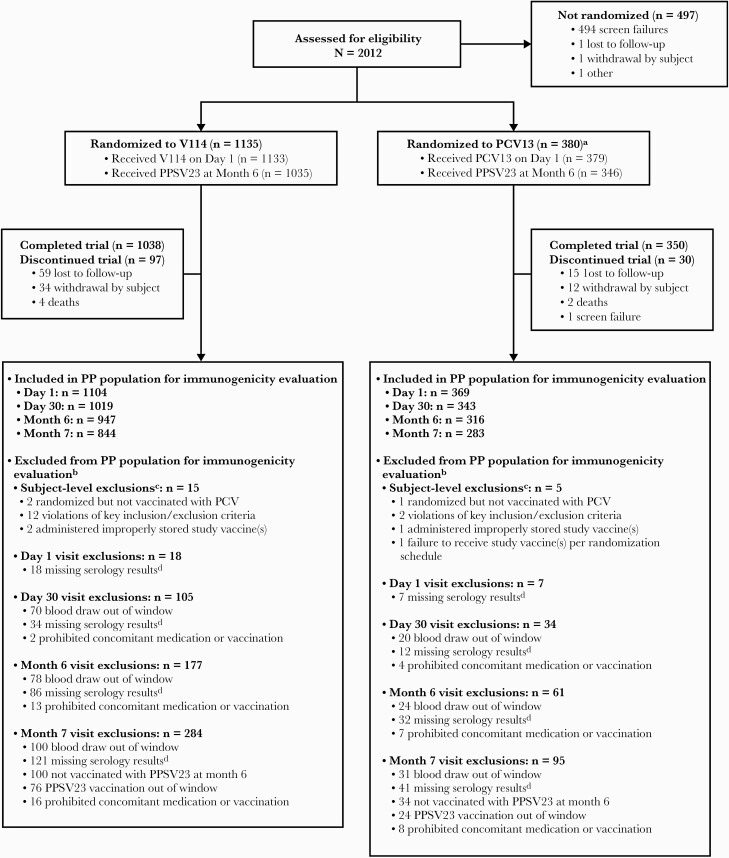
Figure 1.
Participant disposition. Percentages are calculated based on the number of subjects randomized unless otherwise noted. Participants could have been considered to complete the study without receipt of PPSV23. aOne participant in the PCV13 group incorrectly received V114 and was included in the V114 group for safety analyses. bSubjects may have >1 reason for exclusion. Subjects are displayed in all applicable categories. cSubject-level exclusions result in exclusion from analyses at all timepoints. dSubjects who have missing serology results for all 15 serotypes. Reasons for missing serology results may include discontinuation prior to serum sample collection, failure to provide a serum sample, serum sample lost or damaged, and failure to receive PPSV23 prior to a subsequent serum collection. Abbreviations: PCV, pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PP, per-protocol; PPSV23, 23-valent pneumococcal polysaccharide vaccine; V114, 15-valent pneumococcal conjugate vaccine.