Abstract
This case report highlights the initial presentation of Addison’s disease in a 19-year-old individual with coronavirus disease. Coronavirus disease is an infectious disease, which often presents with fever and respiratory and gastrointestinal symptoms. Here, we describe a challenging case of a patient with coronavirus disease, who initially presented with altered mental status, hyponatremia, and cerebral edema, with subsequent workup leading to the diagnosis of Addison’s disease.
Keywords: Addison’s disease, adrenal insufficiency, COVID-19
Introduction
Coronavirus disease (COVID-19) is an infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). This is a contagious disease and is transmitted via respiratory droplets and direct contact. 1 COVID-19 patients often present with respiratory symptoms, generalized fatigue, and fever. Gastrointestinal manifestations like diarrhea, nausea, and vomiting are also not uncommon. The diagnosis of COVID-19 is usually established using nasopharyngeal swab polymerase chain reaction (PCR) test. As the literature continues to evolve, many treatment protocols have been suggested to treat COVID-19, yet no evidence-based definitive treatment is currently available. 2 In this case, it was very unusual for our patient to present with features of adrenal insufficiency without prior history of adrenal disease making the initial diagnosis challenging.
Case report
A 19-year-old female with medical history of Raynaud’s phenomenon arrived at the emergency department (ED) unresponsive. After attending a party 5 days prior, the patient was in her usual state of health. Due to a COVID-19 exposure at the party, the patient tested positive for the disease 4 days before her ED visit. She then developed generalized malaise, nausea, multiple episodes of emesis, and decreased oral intake. On the day of presentation, she was noted to be in an altered mental state, had difficulty with her words, and had an episode of posturing, prompting an urgent request for emergency medical services (EMS).
Upon arrival into the ED, her temperature was 96°F, heart rate 150 beats per minute (bpm), blood pressure (BP) 90/56 mm Hg, oxygen saturation 100% on 15 L non-rebreather mask, and her respiratory rate was 30. Her systemic examination was benign except for a Glasgow Coma Scale (GCS) of 6 for which she was intubated. She received 2 L of isotonic fluids and was started on a norepinephrine infusion due to persistent hypotension. A non-contrast computed tomography (CT) of the head was obtained, showing cerebral edema. Post intubation, her arterial blood gas showed a pH of 6.99, PCO2 of 53 mm Hg, PO2 of 47 mm Hg, and a bicarbonate of 12 mmol/L. Her chest X-ray was unremarkable. The main laboratory investigations at presentation are shown in Table 1.
Table 1.
Laboratory findings of the patient at presentation and during hospitalization.
| Laboratory | Day of admission (day 1) | Day 2 | Day of discharge (day 7) | Normal range |
|---|---|---|---|---|
| WBC count (k/mm3) | 7.0 | 15.1 | 5.0 | 4–11 |
| HB (g/dL) | 12.4 | 12.3 | 10.0 | 11.7–15.5 |
| Platelets (k/mm3) | 309 | 265 | 330 | 150–460 |
| INR | 1.1 | 0.9–1.1 | ||
| D dimer (mg/L FEU) | 0.93 | <1.01 | ||
| Sodium (mmol/L) | 110 | 137 | 141 | 133–145 |
| Potassium (mmol/L) | 6.9 | 4.6 | 3.6 | 3.6–5.2 |
| Glucose (mg/dL) | 63 | 271 | 94 | 70–99 |
| Chloride (mmol/L) | 80 | 108 | 103 | 98–107 |
| HCO3 (mmol/L) | 10 | 12 | 24 | 22–29 |
| Anion gap | 21 | 17 | 14 | 4–17 |
| Urea (mg/dL) | 29 | 23 | 13 | 6–20 |
| Creatinine (mg/dL) | 3.3 | 2 | 0.6 | 0.5–1 |
| AST (U/L) | 40 | 17 | 0–32 | |
| ALT (U/L) | 22 | 24 | 0–32 | |
| Lactate (mmol/L) | 5.1 | 0.5–2.2 | ||
| TSH (uIU/mL) | 22.4 | 2.13 | 0.4–4 | |
| Free T4 (ng/dL) | 1.69 | 1.30 | 0.7–1.80 | |
| CRP (mg/dL) | 24 | 1.6 | 0–0.5 |
WBC: white blood cell; HB: hemoglobin; INR: international normalized ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TSH: thyroid-stimulating hormone; CRP: C-reactive protein.
The bold values in Table 1 are the abnormal values.
Patient was subsequently admitted to the pediatric intensive care unit (PICU) for further management. While in the PICU, she was treated with hypertonic saline. A cortisol level was drawn. Norepinephrine infusion was titrated for goal BP and an intravenous dose of 100 mg of hydrocortisone was administered. Her hyperkalemia was treated with calcium gluconate, insulin, dextrose, and albuterol.
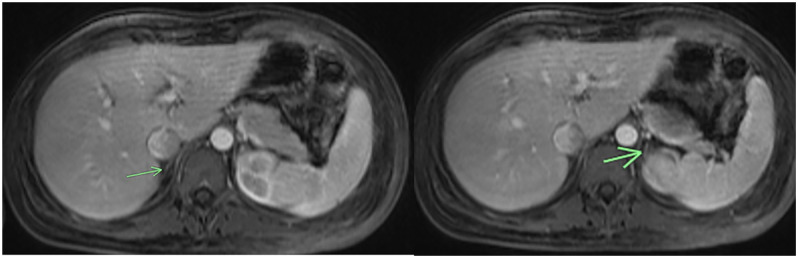
She had significant improvement in her hemodynamics, her lab values improved (Table 1), and she was successfully extubated the next day. Magnetic resonance imaging (MRI) of her abdomen showed both adrenals to be diminutive without any evidence of nodularity or hemorrhage (Figure 1).
Figure 1.
MRI of abdomen showing both adrenal glands (arrow) to be diminutive.
Her thyroid-stimulating hormone (TSH) normalized as expected (Table 1), as high TSH with a normal Free T4 can be due to non-thyroidal acute illness. Adrenocorticotropic hormone (ACTH) and 21-hydroxylase antibody were obtained. Her cortisol level resulted at 1.0 µg/dL. Her ACTH level was normal at 26 pg/mL (normal 6–50 pg/mL), drawn 18 hours after receiving her hydrocortisone dose. Her 21-hydroxylase antibody was positive, confirming the diagnosis of adrenal insufficiency due to Addison’s disease. She was continued on hydrocortisone at 25 mg every 8 hours and subsequently titrated to an oral dose of 35 mg twice daily. Since autoimmune adrenal insufficiency is associated with other autoimmune processes targeting endocrine glands in more than 50% of patients and given the initial increase in TSH described in the patient, thyroid peroxidase antibody and antithyroglobulin antibody were sent. Although her thyroid peroxidase antibody was normal (<3 IU/mL), her antithyroglobulin antibody was high at 20.8 IU/mL (normal < 4.1 IU/mL) raising concerns for autoimmune thyroiditis. As thyroid function tests (TFT) had normalized, she was not started on any thyroid supplementation but was advised to follow up with the endocrinology team for evaluation for other possible endocrine autoimmunity and repeat thyroid function monitoring due to the risk of hypothyroidism in the future.
Once additional history was obtained, she reported salt craving, fatigue, and hyperpigmentation of her buccal mucosa starting 6 months prior. This information was not available at the time of presentation because of her neurological status.
She was discharged on hospital day 7 with oral hydrocortisone at a dose of 25 mg/m2/d which was quickly tapered to 10 mg/m2/d as she returned to her baseline as well as fludrocortisone at 0.1 mg once daily with instructions for stress dosing of her hydrocortisone during illness and follow up with the endocrine team.
Discussion
Although it is well established that intercurrent illness can trigger adrenal decompensation leading to a diagnosis or worsening or primary adrenal insufficiency, this is the first description of a case in which SARS-CoV-2 infection/COVID-19 unmasks Addison’s disease. Given the reported actions of the virus on hypothalamic/pituitary adrenal signaling, it is interesting to consider possible mechanisms for the adrenal decompensation in this setting.
The virus is responsible for a plethora of organ involvement, not just limited to respiratory system. The angiotensin-converting enzyme-2 (ACE2) receptor on multiple organ system cells has been recognized as its point of entry targeted by the spike protein on the viral surface. 3 This receptor is found in respiratory epithelial cells, myocardium, thyroid, pancreatic islets, adrenal gland, and hypothalamus among others.
The direct and indirect effects of the virus on different tissues are being described over time. By targeting the renin–angiotensin–aldosterone system (RAAS) through occupancy of ACE2 receptor, the downstream effect of aldosterone in sodium preservation is affected and the negative feedback effect of ACE2 in regulation of the axis is blocked. The direct targeting of ACE2 receptor on hypothalamus and pituitary gland potentially blocks the release of ACTH and corticotropin-releasing hormone (CRH). The tumor necrosis factor-alpha (TNF-alpha) in the cytokine storm during the systemic illness can also have an inhibitory effect on ACTH release, further exacerbating the lack of necessary stimulation required by the adrenals to respond. 4 By these mechanisms, the virus achieves an immune invasive strategy to survive and causes more damage, necessitating the requirement of hydrocortisone and vasopressor support.
Another mechanism of influence on the adrenal glands is the procoagulant effect where microthrombi blocks the vasculature of adrenals leading to ineffective mineralocorticoid and glucocorticoid release. 5
Our patient presented in a state of shock with COVID-19. Although lab workup pointed toward an adrenal cause, her initial history, as given by her parents, did not reveal any prior steroid use, infections, surgeries, hyperpigmentation, or hospitalizations making the initial diagnosis less obvious. Her rapid improvement after the initiation of steroids and the subsequent laboratory data helped diagnose her primary adrenal insufficiency or Addison’s disease. Feedback signaling normally causes the lowered cortisol production by autoimmune damaged adrenals to elevate CRH and ACTH. We postulate that interference by SARS-CoV-2 with the normal feedback loops by mechanisms detailed above may have resulted in our patient having a less elevated ACTH level than classically described in primary adrenal insufficiency, which lead to further adrenal decompensation.
Making the diagnosis and initiating therapy with stress dose glucocorticoids followed by special attention to glucocorticoid management over the acute and possible long-haul course of COVID-19 illness have been previously described. 6
Conclusion
As the COVID-19 pandemic continues to spread, it is important for clinicians caring for acutely ill patients to be aware of unusual patterns of presentation and possibility of comorbid conditions. This case illustrates that adrenal insufficiency should be considered as a diagnostic possibility in hemodynamically unstable patients presenting with COVID-19 even without any prior history of adrenal disease. To our knowledge, this is the first case of previously undiagnosed primary adrenal disease presenting during concurrent COVID-19 infection. This case offers insight into the spectrum of COVID presentation including serious and rare medical conditions such as adrenal insufficiency that may be unmasked by COVID infection, complicating the diagnostic and treatment process.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article. Subject had decisional capacity to provide written consent.
ORCID iD: Pallav Bhattarai  https://orcid.org/0000-0002-2033-3262
https://orcid.org/0000-0002-2033-3262
References
- 1. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterization protocol: prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med 2020; 288(2): 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McLachlan CS. The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin Hypertens 2020; 26: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Natarajan R, Ploszaj S, Horton R, et al. Tumor necrosis factor and interleukin-1 are potent inhibitors of angiotensin-II-induced aldosterone synthesis. Endocrinology 1989; 125(6): 3084–3089. [DOI] [PubMed] [Google Scholar]
- 5. Raekha K, Guruparan T, Siddiqi S, et al. A case of adrenal infarction in a patient with COVID 19 infection. BJR Case Rep 2020; 6(3): 20200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isidori AM, Arnaldi G, Boscaro M, et al. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J Endocrinol Invest 2020; 43(8): 1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]