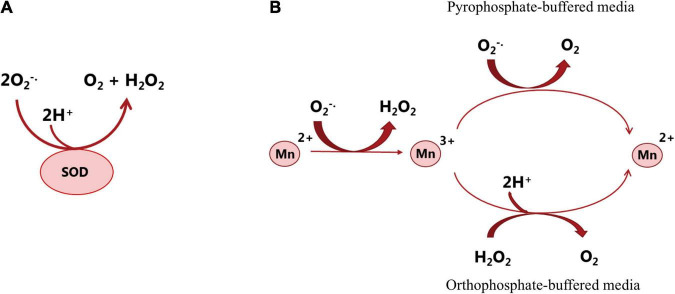
FIGURE 3.
Dismutation of superoxide (O2–) by superoxide dismutase (A) or manganese (B). SOD reacts with O2– and generates H2O2 and O2 that are less reactive (Melo et al., 2011). Studying the catalytic activity of manganese in different in vitro systems (in pyrophosphate or orthophosphate-buffered media) showed that in both situations, Mn2+ is oxidized by O2–, which forms Mn3+ and H2O2 and then the formed Mn3+ is re-reduced to Mn2+, and O2 is produced. In the presence of pyrophosphate, O2– acts as both oxidizing and reducing agent; in the presence of orthophosphate or some other buffered media, another reductant such as NAD(P)H or H2O2 is required for the scavenging activity of manganese. The above-mentioned mechanisms potentially occur in the bacterial cell. However, the reaction rate and the reaction course are determined by the ligands that are available and can associate with manganese (Archibald and Fridovich, 1981).