Abstract
Purpose
We report a case of a 19-year-old male who presented with bilateral Vogt-Koyanagi-Harada (VKH)-like panuveitis following an injection of an inactivated Covid-19 vaccine.
Observations
A 19-year-old male was referred to our clinic with a 2-week history of blurred vision on both eyes and headaches, 12 hours following the administration of the first dose of an inactivated Covid-19 virus vaccine (Sinovac). He denied any past ocular or medical history. Clinical examination and multimodal imaging tests identified serous retinal detachment and choroidal thickening posteriorly and deep yellow foci in the far peripheral retina. Aqueous humor analysis ruled out viral and bacterial infection including Covid-19, but demonstrated an elevated interleukin-6 level. A workup ruled out systemic infection or autoimmune disease. Although the patient received a single positive T-SPOT result, no other clinical evidence supported active tuberculosis infection. Non-infectious panuveitis was diagnosed and treated with periocular steroids that quickly resolved the serous retinal detachment.
Conclusions and Importance
This is the first report of VKH-like uveitis following an inactivated Covid-19 vaccine, with aqueous humor analysis ruling out viral or bacterial infection and demonstrating an elevated interleukin-6 level. Though rare, VKH-like uveitis may be associated with administration of an inactivated Covid-19 vaccine.
Keywords: Covid-19, Vogt–Koyanagi–Harada disease, Uveitis, Inactivated vaccine
1. Introduction
The Covid-19 pandemic has promoted vaccine development at a fast pace. There are currently over 10 vaccines developed and approved for use within 2 years of the pandemic.1 Since the implementation of vaccine campaigns, reports of ocular adverse effects have emerged. Although uveitis is a rare adverse event, there are reported events following various Covid-19 vaccines2, 3, 4, 5, 6, 7, 8, 9 including anterior uveitis, multiple evanescent white dot syndrome (MEWDS), acute macular neuroretinopathy, central serous retinopathy, multifocal choroidtis, and reactivation of Vogt-Koyanagi-Harada (VKH) disease.
VKH-like uveitis is a very rare adverse event of vaccination, and only several cases have been reported worldwide following vaccines against yellow fever, influenza, hepatitis B, and human papilloma virus (HPV), as well as following administration of the bacille Calmette-Guerin (BCG) vaccine.10, 11, 12, 13 Recently, cases of VKH disease following Covid-19 mRNA and adenovirus vector vaccine have been reported.14 We report a case of bilateral VKH-like disease following an inactivated Covid-19 virus vaccine.
2. Case report
A 19-year-old male was referred to our clinic with a two-week history of bilateral eye redness and blurred vision. The patient denied any ocular or medical history. The patient had received the first dose of an inactivated Covid-19 virus vaccine two weeks prior, after which the patient reported headaches and fatigue 2 h later and subsequently developed bilateral blurred vision 12 hours following the vaccination. On examination, the best visual acuity was 20/20 bilaterally and intraocular pressure was 11.2 mm Hg in the right eye and 11.3 mm Hg in the left eye. Anterior segment examination revealed mild conjunctival congestion, gray fine keratic precipitates, and 2+ cells in the anterior chamber in both eyes. Slit lamp examination revealed 1+ cells in the anterior vitreous. Dilated indirect ophthalmoscopic examination revealed 1+ vitreous haze and demonstrated serous retinal detachments and multiple choroidal foci far peripherally in both eyes (Fig. 1), which were further confirmed by optical coherence tomography (OCT) (Fig. 2). Autofluorescence (AF) was unremarkable (Fig. 2). Wide-field fluorescein angiography (FA) showed mild hyper-fluorescence in the posterior pole and multiple hyperfluorescent foci inferiorly, more prominent in the left eye (Fig. 3). Aqueous humor was sent for viral and bacterial detection including cytomegalovirus, rubella virus, herpes simplex virus as well as Covid-19 virus, but all yielded negative results. However, inflammatory analysis demonstrated elevated interleukin-6 (IL-6) in both eyes (1024.0 pg/ml OD and 734.6 pg/ml OS). Systemic investigation was unremarkable, including cranial magnetic resonance imaging, complete blood count, serum rheumatoid factor, antinuclear antibodies, and antineutrophilic cytoplasmic antibodies. Serologic tests for the Covid-19 virus, hepatitis B, herpes simplex virus, human immunodeficiency virus, and syphilis were negative. Although the patient's interferon gamma release assay (T-SPOT) results were positive, chest X-ray and computed tomography were normal, along with IL-6, C-reactive protein, and erythrocyte sedimentation rate. Other positive results were anti-cytomegalovirus, rubella virus and anti-herpes simplex virus IgG antibodies, but not IgM.
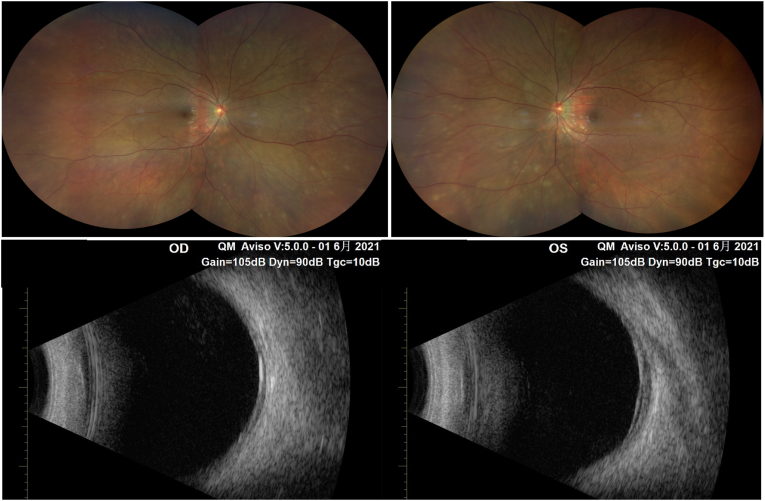
Fig. 1.
Ultra-widefield fundus photograph (upper) and ultrasound B-scan (lower) showing multiple serous retinal detachments and multiple choroidal foci in the far periphery.
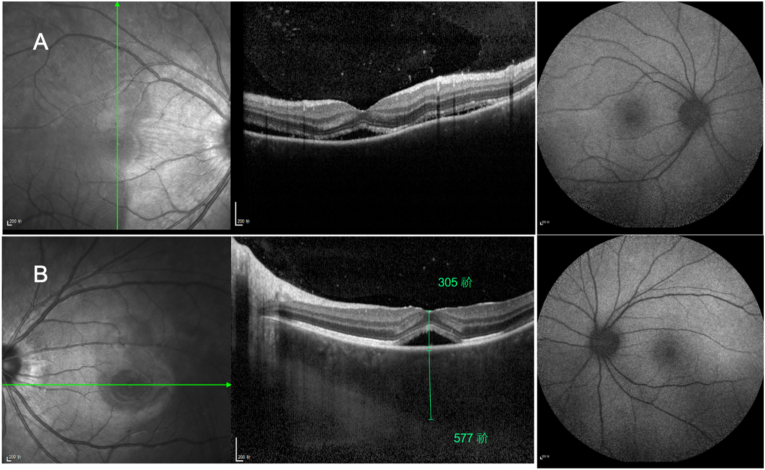
Fig. 2.
Combined optical coherence tomography and autofluorescence of eyes with uveitis showing serous detachment of the neurosensory retina and choroidal thickening in the central parafoveal macula.
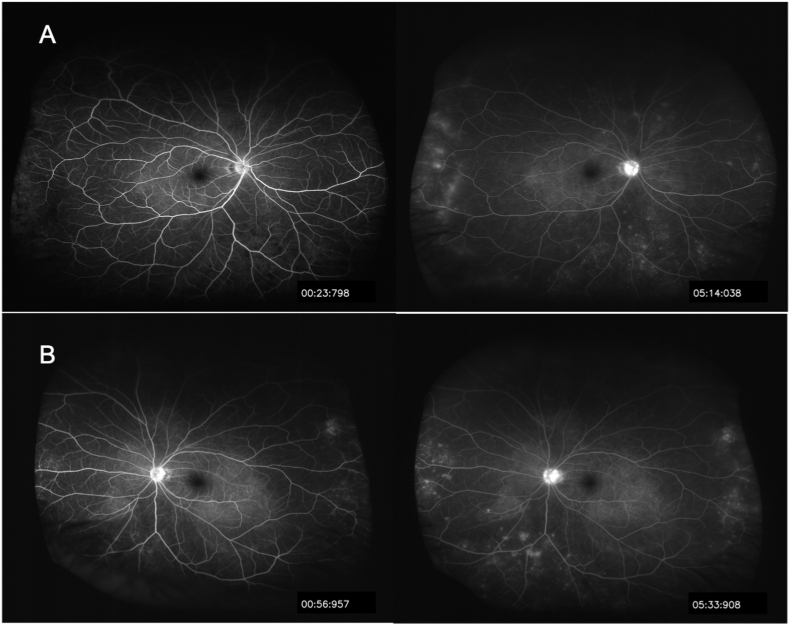
Fig. 3.
Ultra-widefield fluorescein angiography showing multiple hyperfluorescent spots and choroidal hyperfluorescent foci inferiorly in the late phase (right column).
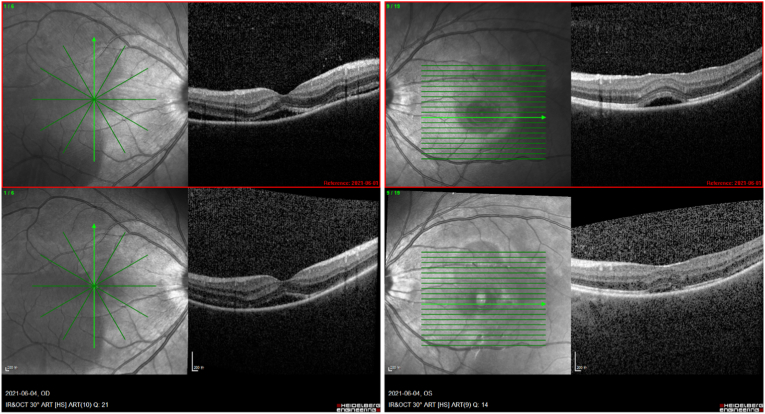
After initial evaluation, the patient was treated for non-infectious panuveitis with periocular injections of triamcinolone acetonide 40 mg in each eye. Subretinal fluid decreased quickly within two days of treatment, (Fig. 4). Blurred vision resolved in five days, and the patient has had no recurrent ocular disturbance through four months of follow-up.
Fig. 4.
Follow-up optical coherence tomography of the patient after treatment with periocular steroids showing decreased subretinal fluid (lower) compared with baseline (upper).
3. Discussion
Our case highlights the possibility of panuveitis following administration of an inactivated Covid-19 vaccine. While the rapid resolution of the disease with excellent visual recovery reassured us that this type of adverse event could be transient and treatable, the mechanism underlying vaccine-associated uveitis is unclear, and it is difficult to prove causality in these rare cases. Commonly proposed mechanisms of inflammation may be induced by the adjuvants that are routinely used in inactivated or subunit vaccines to enhance the immunogenicity of the virus.15 The autoimmunity has been classified as Shoenfeld syndrome that is often accompanied by arthralgia and fatigue, typically occurring in patients with an autoimmune disorder. Like our case, VKH-like uveitis is reported to occur mostly in association with the inactivated vaccinates against yellow fever, influenza, BCG and HPV.10, 11, 12, 13 However, there are reported VKH diseases related to Pfizer mRNA as well as the adenovirus vector vaccine (AZD1222). Reactivation of VKH disease after the second dose of Pfizer mRNA (BNT162b2) has also been reported.8 Both of these vaccines do not utilize an additional adjuvant.8,14 Due to the similar phenotype of uveitis despite a different type of vaccine, antigen mimicry from the virus could be a potential trigger. In this case, additional aqueous humor analysis ruled out intraocular infection and demonstrated the inflammatory status, with prominent elevated IL-6. More analyses are needed to elucidate the mechanism of this reaction.
Although we called this case VKH-like uveitis due to the inflammatory nature and multiple serous detachments of the neurosensory retina, the OCT did not show features like subretinal septa or folds of the retinal pigmented epithelium as are often seen in VKH.16 The FA of this patient showed mild and even hyperfluorescence in the area of retinal detachment, unlike multifocal leaks at the level of the retinal pigment epithelium that pool into the sub-neurosensory retinal space in VKH syndrome.17,18 There are some deep focal lesions in the mid-periphery in both eyes that are consistent with the hyperfluorescent spot on the FA. However, there are no positive findings in the AF or OCT, distinguishing this condition from MEWDS.19, 20, 21 Given the atypical combined features in this case, the close temporal relationship between the symptom onset and the vaccination, the immunocompetent status of this patient, no evidence of infection, and quickly improvement in vision following treatment with steroids, the vaccine can be categorized as a probable trigger, according to the Naranjo adverse event probability scale.22 Nevertheless, a causal relationship cannot be established from this single case report.
4. Conclusions
As the urge for a vaccination against Covid-19 continues, potential ocular adverse events should be reported in detail to increase awareness among the medical community.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and use of any accompanying images.
Declaration of competing interest
No funding or grant support was provided for this report.
All the authors have no financial disclosures: XJC BW XXL.
All the authors attest that they meet the current ICMJE criteria for authorship.
Acknowledgments
None.
References
- 1.Ng X.L., Betzler B.K., Testi I., et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021:1–9. doi: 10.1080/09273948.2021.1976221. [published Online First: 2021/09/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinovitch T., Ben-Arie-Weintrob Y., Hareuveni-Blum T., et al. Uveitis following the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association. Retina. 2021 doi: 10.1097/iae.0000000000003277. [published Online First: 2021/08/10] [DOI] [PubMed] [Google Scholar]
- 3.Mudie L.I., Zick J.D., Dacey M.S., et al. Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. 2021;29(4):741–742. doi: 10.1080/09273948.2021.1949478. [published Online First: 2021/07/03] [DOI] [PubMed] [Google Scholar]
- 4.ElSheikh R.H., Haseeb A., Eleiwa T.K., et al. Acute uveitis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021:1–3. doi: 10.1080/09273948.2021.1962917. [published Online First: 2021/08/12] [DOI] [PubMed] [Google Scholar]
- 5.Fowler N., Mendez Martinez N.R., Pallares B.V., et al. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. Am J Ophthalmol Case Rep. 2021;23 doi: 10.1016/j.ajoc.2021.101136. [published Online First: 2021/06/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal M., Murthy S.I., Annum S. Bilateral multifocal choroiditis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(4):753–757. doi: 10.1080/09273948.2021.1957123. [published Online First: 2021/08/05] [DOI] [PubMed] [Google Scholar]
- 7.Mambretti M., Huemer J., Torregrossa G., et al. Acute macular neuroretinopathy following coronavirus disease 2019 vaccination. Ocul Immunol Inflamm. 2021;29(4):730–733. doi: 10.1080/09273948.2021.1946567. [published Online First: 2021/07/01] [DOI] [PubMed] [Google Scholar]
- 8.Papasavvas I., Herbort C.P., Jr. Reactivation of Vogt-Koyanagi-Harada disease under control for more than 6 years, following anti-SARS-CoV-2 vaccination. J Ophthalmic Inflamm Infect. 2021;11(1):21. doi: 10.1186/s12348-021-00251-5. [published Online First: 2021/07/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichi F., Aljneibi S., Neri P., et al. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139(10):1131–1135. doi: 10.1001/jamaophthalmol.2021.3477. [published Online First: 2021/09/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogan B., Erol M.K., Cengiz A. Vogt-Koyanagi-Harada disease following BCG vaccination and tuberculosis. SpringerPlus. 2016;5 doi: 10.1186/s40064-016-2223-4. 603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M. Vogt-Koyanagi-Harada Syndrome following influenza vaccination. Indian J Ophthalmol. 2016;64(1):98. doi: 10.4103/0301-4738.178141. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sood A.B., O'Keefe G., Bui D., et al. Vogt-koyanagi-harada disease associated with hepatitis B vaccination. Ocul Immunol Inflamm. 2019;27(4):524–527. doi: 10.1080/09273948.2018.1483520. [published Online First: 2018/06/29] [DOI] [PubMed] [Google Scholar]
- 13.Dogan B., Erol M.K., Cengiz A. Vogt-Koyanagi-Harada disease following BCG vaccination and tuberculosis. SpringerPlus. 2016;5:603. doi: 10.1186/s40064-016-2223-4. [published Online First: 2016/06/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koong L.R., Chee W.K., Toh Z.H., et al. Vogt-koyanagi-harada disease associated with COVID-19 mRNA vaccine. Ocul Immunol Inflamm. 2021:1–4. doi: 10.1080/09273948.2021.1974492. [published Online First: 2021/09/11] [DOI] [PubMed] [Google Scholar]
- 15.Watad A., Quaresma M., Brown S., et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld's syndrome) - an update. Lupus. 2017;26(7):675–681. doi: 10.1177/0961203316686406. [published Online First: 2017/01/07] [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi Y., Otani T., Kishi S. Tomographic features of serous retinal detachment with multilobular dye pooling in acute Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2007;144(2):260–265. doi: 10.1016/j.ajo.2007.04.007. [published Online First: 2007/05/30] [DOI] [PubMed] [Google Scholar]
- 17.Rao N.A., Gupta A., Dustin L., et al. Frequency of distinguishing clinical features in Vogt-Koyanagi-Harada disease. Ophthalmology. 2010;117(3):591–599. doi: 10.1016/j.ophtha.2009.08.030. 99.e1. [published Online First: 2009/12/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodhi S.A., Reddy J.L., Peram V. Clinical spectrum and management options in Vogt-Koyanagi-Harada disease. Clin Ophthalmol. 2017;11:1399–1406. doi: 10.2147/opth.S134977. [published Online First: 2017/08/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee G.E., Lee B.W., Rao N.A., et al. Spectral domain optical coherence tomography and autofluorescence in a case of acute posterior multifocal placoid pigment epitheliopathy mimicking Vogt-Koyanagi-Harada disease: case report and review of literature. Ocul Immunol Inflamm. 2011;19(1):42–47. doi: 10.3109/09273948.2010.521610. [published Online First: 2010/11/03] [DOI] [PubMed] [Google Scholar]
- 20.Gass J.D. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol. 1968;80(2):177–185. doi: 10.1001/archopht.1968.00980050179005. [published Online First: 1968/08/01] [DOI] [PubMed] [Google Scholar]
- 21.Yeh S., Forooghian F., Wong W.T., et al. Fundus autofluorescence imaging of the white dot syndromes. Arch Ophthalmol. 2010;128(1):46–56. doi: 10.1001/archophthalmol.2009.368. [published Online First: 2010/01/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naranjo C.A., Busto U., Sellers E.M., et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [published Online First: 1981/08/01] [DOI] [PubMed] [Google Scholar]