Abstract
Aims
Although pre-hospital risk stratification of patients with suspected non-ST-elevation acute coronary syndrome (NSTE-ACS) by ambulance paramedics is feasible, it has not been investigated in daily practice whether referral decisions based on this risk stratification is safe and does not increase major adverse cardiac events (MACE). In Phase III of the FamouS Triage study, it was investigated whether referral decisions by ambulance paramedics based on a pre-hospital HEART score, is non-inferior to routine management.
Methods and results
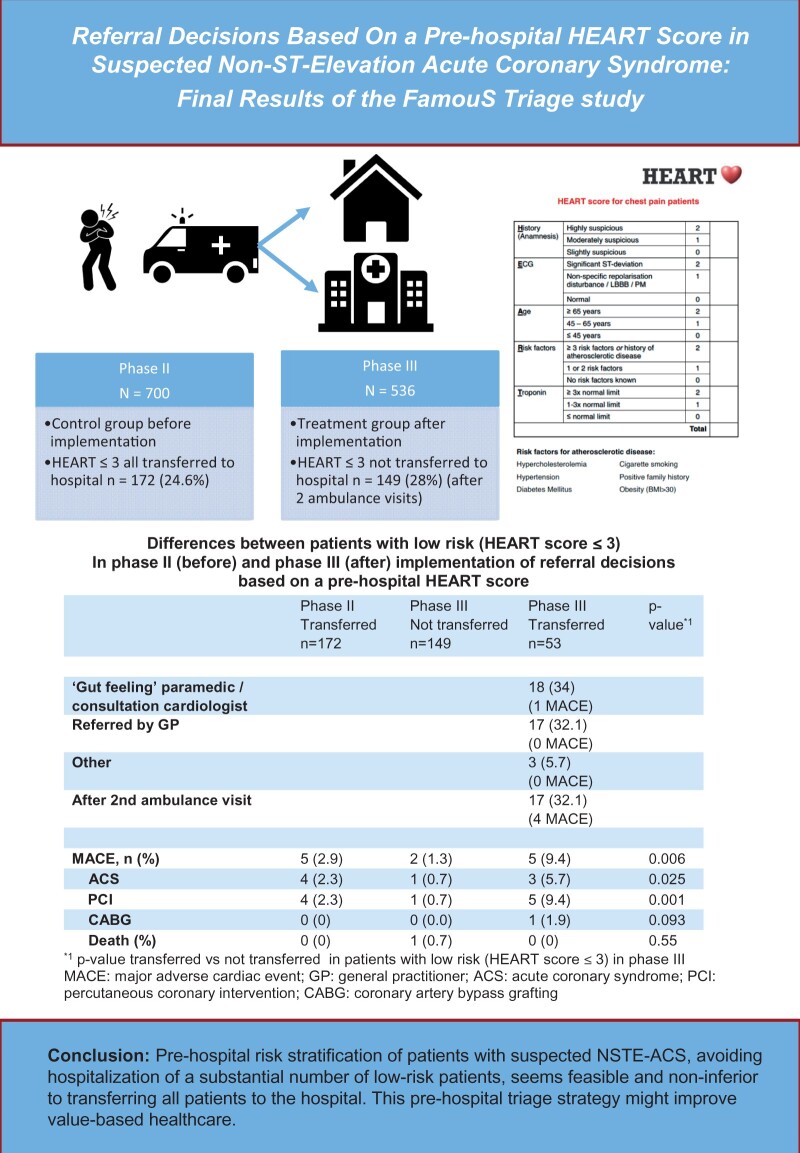
FamouS Triage Phase III is a non-inferiority study, comparing the occurrence of MACE before (Phase II) and after (Phase III) implementation of referral decisions based on a pre-hospital HEART score. In Phase II, all patients were risk-stratified and referred to the hospital; in Phase III, low-risk patients (HEART score ≤ 3) were not referred. Primary endpoint was MACE (acute coronary syndrome, revascularization, or death) within 45 days. A total of 1236 patients were included. Mean age was 63 years, 43% were female, 700 patients were included in the second phase and 536 in the third phase in which 149 low-risk patients (28%) were not transferred to the hospital. Occurrence of 45 days MACE was 16.6% in Phase II and 15.7% in Phase III (P = 0.67). Percentage MACE in low-risk patients was 2.9% in Phase II and 1.3% in Phase III. After adjustments for differences in baseline variables, the hazard ratio of 45 days MACE in Phase III was 0.88 (95% confidence interval 0.63–1.25) as compared to Phase II.
Conclusion
Pre-hospital risk stratification of patients with suspected NSTE-ACS, avoiding hospitalization of a substantial number of low-risk patients, seems feasible and non-inferior to transferring all patients to the hospital.
Keywords: Pre-hospital, Triage, Acute-Coronary-Syndrome, HEART score
Graphical Abstract
Introduction
Emergency department (ED) overcrowding is worldwide an increasing challenge, leading to increased length of stay, high costs and reduced patient satisfaction. A substantial part, approximately 10% of all ED admissions, consists of patients with chest pain, suspected for non-ST-elevation acute coronary syndrome (NSTE-ACS).1 About 65% of these patients are observed for hours or even hospitalized for further diagnostics, despite the fact that about 80% are at low risk and do not have an acute coronary syndrome.2–4 Several studies showed that additional diagnostics in low-risk patients lead to a longer length of stay and higher costs without reducing clinical events.5–8
In patients with suspected NSTE-ACS, pre-hospital risk assessment is feasible and has comparable accuracy to in-hospital risk assessment.9–13 History, ECG, Age, Risk factors, and Troponin (HEART) score calculation (Table 1), including point-of-care (POC) troponin assessment can be used pre-hospitally by paramedics.9,13–15 It adequately stratifies patients in low-, intermediate-, and high risk for major adverse cardiac events (MACE).2,10,16 It has, however, not yet been studied in daily practice whether selected low-risk patients with suspected NSTE-ACS can safely stay at home based on pre-hospital risk assessment, avoiding ED presentation.
Table 1.
Calculation of the HEART score
| HEART | ||
| History (anamnesis) | Highly suspicious | 2 |
| Moderately suspicious | 1 | |
| Slightly suspicous | 0 | |
| ECG | Significant ST-segment deviation | 2 |
| Non-specific repolarization disturbance/LBBB/PM | 1 | |
| Normal | 0 | |
| Age | ≥65 years | 2 |
| >45 and <65 years | 1 | |
| ≤45 years | 0 | |
| Risk factorsa | ≥3 risk factors or history of atherosclerotic disease | 2 |
| 1 or 2 risk factors | 1 | |
| No known risk factors | 0 | |
| Troponin | ≥3× normal limit | 2 |
| 1–2× normal limit | 1 | |
| Normal limit or lower | 0 | |
| Total | ||
ECG, electrocardiogram; HEART, History, ECG, Age, Risk factors, and initial Troponin; LBBB, left bundle branch block; PM, pacemaker.
Risk factors: hypercholesterolaemia, hypertension, diabetes mellitus, cigarette smoking, family history of atherosclerotic disease, BMI >30 kg/m2.
In Phase II of the FamouS Triage study, the pre-hospital acquired HEART score was incorporated in routine patient assessment, but without treatment consequences.10,17 Phase III of the FamouS Triage study is the first study in which paramedics used a pre-hospital acquired HEART score for referral decisions (low-risk patients were not transferred). The current analyses compare MACE within 45 days between Phase II (all patients transferred) and Phase III (low-risk patients observed at home).
Methods
Study design
FamouS Triage is a non-inferiority, controlled before-after multicentre study with a sequential design with the aim to assess feasibility and safety of pre-hospital risk assessment by paramedics using the HEART score. The design has been described previously.18 The study was performed in two hospitals in The Netherlands (Deventer Hospital and Isala hospital) and 33 emergency medical services vehicles from 2 regional ambulance services (Ambulance IJsselland and Witte Kruis ambulancezorg) staffed by approximately 110 paramedics which are registered nurses, specialized in pre-hospital care. In FamouS Triage II,17 a pre-hospital HEART score including POC troponin was prospectively assessed by paramedics without treatment consequences. These patients formed the control (before) group. In the treatment (after) group, a HEART score was calculated in the same way, but patients with a HEART score of ≤3 were asked to give informed consent to be observed at home instead of being transferred to the hospital. In those patients, a second HEART score was assessed at home 3–12 h after inclusion. The reason for a second reassessment was that previous results showed that patients who were included shortly after onset of symptoms might have false negative troponin results.17 When patients were not referred, their general practitioner was informed and patients were instructed by the paramedic to contact their general practitioner to investigate the cause of their complaints, particularly when complaints persisted. Patients with a HEART score of >3 were transferred to a nearby hospital.
Regulation statement
This study was conducted according to the principles of the current declaration of Helsinki and in accordance with Dutch law on Medical Research Involving Human Subjects Act (WMO). The study was approved by the Institutional Review Board (medical ethical committee of the Isala clinics, Zwolle, the Netherlands, METC No.170526), and subsequently by the boards of the participating hospitals.
Point-of-care troponin
A cardiac troponin T assay was performed on site using the Roche CARDIAC POC troponin T test on the cobas h 232 POC system with a limit of detection of 40–2000 ng/L. The device is able to work properly in a temperature range from 18°C to 32°C, a relative humidity of 10–85% (no condensation) and maximum altitude of 4300 m. The POC testing strips are sustainable for 7 days after removal from the refrigerator. POC test results are available in 8–12 min.
An outcome of 40 ng/L with this assay is comparable to 40 ng/L of a high-sensitivity cardiac troponin T assay with a 99th percentile of 14 ng/L. This means that a positive result on the POC device will have a value of 40 ng/L or higher. Therefore, all patients with a positive POC result immediately received two points in the HEART score on the ‘Troponin’ element. Patients with a positive POC troponin result were transferred to the hospital whether or not the total HEART score was ≤3.
Study population
The inclusion criteria were out-of-hospital patients aged ≥18 years visited by an ambulance with a pre-hospital suspicion of NSTE-ACS. The exclusion criteria were electrocardiographic ST-elevation, pregnancy, comatose state, cognitive impairment, shock, cardiac asthma, ventricular tachyarrhythmia, end-stage renal disease, an obvious non-cardiac cause for complaints, or a strong suspicion of either aortic dissection or pulmonary embolism.
Study hypothesis and endpoints
The hypothesis was that referral decision based on the pre-hospital HEART score in suspected NSTE-ACS is feasible and non-inferior to routine management according to the current guidelines19 with regards to the occurrence of MACE within 45 days. MACE consisted of the following events: myocardial infarction, unstable angina, percutaneous coronary intervention (PCI), coronary artery bypass grafting, and death by all causes. Adjudication of the final diagnosis was performed by applying current guidelines and the third universal definition of myocardial infarction.19–22 ST-segment elevated myocardial infarction (STEMI) was determined by paramedic ECG judgement. Non-STEMI (NSTEMI) diagnosis was adjudicated when high-sensitive cardiac troponin value was above the 99th percentile upper reference limit (URL) with a significant delta (≥20%) as well as a clinical setting consistent with myocardial ischaemia. Unstable angina was diagnosed when there was a clinical setting consistent with myocardial ischaemia but if high-sensitive cardiac troponin was normal or above the 99th percentile URL without a significant delta. All cases with possible endpoints were reviewed by two independent adjudicators, without knowledge of the HEART score. In case of disagreement, the case was discussed in a plenary adjudication committee meeting, composing at least two cardiologists that were not related to the study, until consensus was reached.
Follow-up
A follow-up duration of 45 days was chosen because the HEART score was validated previously to predict MACE within 6 weeks. Any information that indicated possible endpoints were further investigated through hospital charts and information obtained from general practitioners.
Statistical analysis
Primary analysis
Primary analysis aimed to study whether pre-hospital HEART management will not cause more MACE than routine management. The primary outcome was the absolute difference in 45 days MACE incidence between the control (before) and treatment (after) group.
Secondary analyses
Because of the design of the study, there may be differences in baseline characteristics partly due to a discrepancy in the time of enrolment. Hence, we have to account for potential confounding. To adjust for potential confounders, multivariable analyses were performed by a Cox regression model, with calculating hazard ratios (HRs) with 95% confidence intervals (CIs).
Sample size
The aim of this study was to assess whether pre-hospital referral decisions (treatment group) according to the HEART score is feasible and does not lead to an increase in MACE within 45 days of presentation compared to routine management (control group). Our sample size calculation was therefore based on demonstrating that the proportion of MACE in the treatment group is non-inferior to the proportion observed in the control group. Preliminary results of the second phase of FamouS Triage showed within 45 days 15.7% (95% CI, 13.1–18.6) MACE,17 and we used the expected incidence of 15.7% as the point estimate (meaning no difference between control and intervention) and set the non-inferiority-margin at 7.5% which means that the upper limit of the one-sided 97.5% CI of the difference in MACE between Phases II and III is not above 7.5%. This is comparable to other research.23–27 If there is truly no difference in incidence of MACE between the control and treatment groups, a total of 990 patients was required to be 90% sure that the upper limit of a one-sided 97.5% CI (or equivalently a 95% two-sided CI) would exclude a difference in favour of the control group.28,29 The expected number of patients with loss of follow-up or missing data was estimated to be 10%. Therefore, the total sample size had to be at least 1090 (545 in each group). The control group, composed of FamouS Triage Phase II, consisted of 700 participants. In Phase III, the intention was to include at least 545 participants.
Results
FamouS Triage II (before implementation of HEART score-based referral decisions) was performed from January 2016 until July 2017. During this period, 700 patients were included. A detailed description was published earlier.17
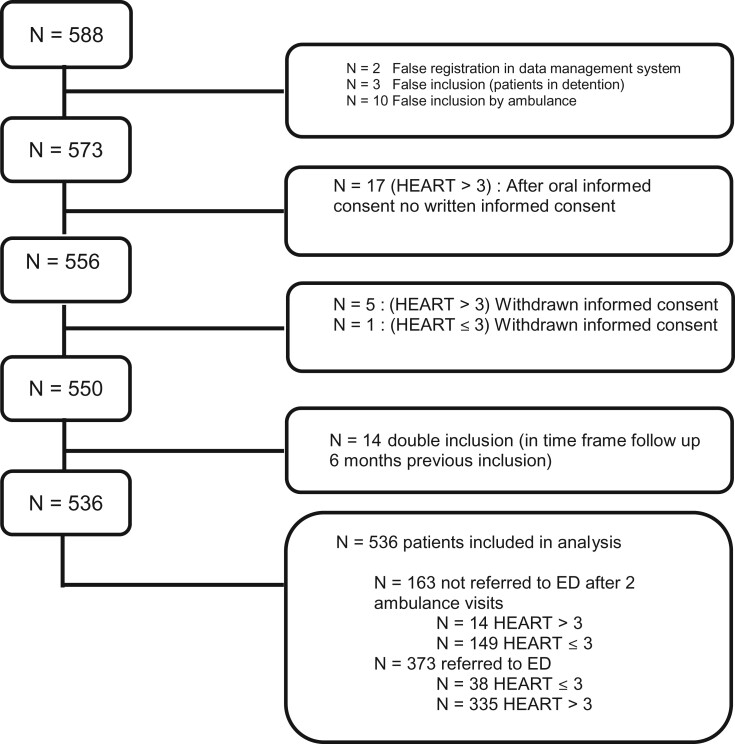
In FamouS Triage III, 588 participants were included from September 2018 to May 2020 (Figure 1). Fifty-two patients (8.8%) were excluded from the analysis because of false registration procedure (n = 2), detention (n = 3), absence of written informed consent (n = 17), withdrawn informed consent (n = 6), double inclusion (in time frame follow-up 6 months previous inclusion, n = 14), leaving 536 patients to be analysed in Phase III.
Figure 1.
Flow chart of patient inclusion in Phase III.
Together, a total of 1236 patients were included in Phases II and III. Mean age was 63 years, 43% were female. Differences in baseline characteristics are summarized in Table 2.
Table 2.
Comparison of baseline characteristics of patients included in Phase II (FT2) or Phase III (FT3)
| Characteristics | FT2 | FT3 | P-value |
|---|---|---|---|
| n = 700 (%) | n = 536 (%) | ||
| Demographics | |||
| Mean age, years (SD) | 63.6 (13.6) | 62.9 (14.7) | 0.13 |
| Male, n (%) | 401 (57.3) | 310 (57.8) | 0.85 |
| Cardiac risk factors, n (%) | |||
| Diabetes mellitus | 120 (17.1) | 74 (13.8) | 0.07 |
| Obesity (body mass index ≥ 30 kg/m2) | 140 (20.0) | 62 (11.6) | 0.01 |
| Hypercholesterolaemia | 275 (39.3) | 132 (24.6) | 0.01 |
| Hypertension | 372 (53.1) | 230 (42.9) | 0.01 |
| Positive family history | 324 (46.3) | 188 (35.1) | 0.01 |
| Current smoking | 156 (22.3) | 117 (21.8) | 0.62 |
| Mean HEART score (SD) | 4.7 (1.7) | 4.3 (1.9) | 0.01 |
| HEART score >3 | 528 (75.4) | 334 (62) | 0.01 |
HEART, History, ECG, Age, Risk factors, and initial Troponin.
In FamouS Triage III, a total of 149 low-risk patients (28%) were not transferred to the hospital. The HEART score of these 149 patients was 0 (2%), 1 (17.4%), 2 (27.5%), and 3 (53%). Of the 387 patients who were transferred to the hospital, 53 patients (13.7%) had a HEART score ≤3: 0 (1, 0.3%) 1 (4, 1.0%), 2 (12, 3.1%), and 3 (36, 9.3%). In Table 3 differences between patients in Phase III who were transferred or not transferred to the hospital are summarized. Transferred patients were older, more often male, and had more often diabetes and a history of hypercholesterolaemia.
Table 3.
Differences in baseline characteristics of 149 low-risk patients who were not transferred to the hospital compared to the transferred patients included in Phase III
| Characteristics | Not transferred | Transferred | P-value |
|---|---|---|---|
| n = 149 | n = 387 | ||
| Demographics | |||
| Mean age, years (SD) | 53.8 (14.6) | 66.4 (13.2) | 0.01 |
| Male, n (%) | 75 (50.3) | 235 (60.7) | 0.03 |
| Cardiac risk factors, n (%) | |||
| Diabetes mellitus | 8 (5.4) | 66 (17.1) | 0.01 |
| Obesity (body mass index ≥ 30 kg/m2) | 14 (9.4) | 48 (12.4) | 0.33 |
| Hypercholesterolaemia | 20 (13.4) | 112 (28.9) | 0.01 |
| Hypertension | 69 (46.3) | 161 (41.7) | 0.34 |
| Positive family history | 49 (32.9) | 139 (35.9) | 0.51 |
| Current smoking | 39 (26.2) | 78 (20.2) | 0.13 |
Fourteen patients (2.6%) with a HEART score >3 were, in most cases after consultation with the cardiologist in the hospital, not transferred to the hospital.
Clinical endpoints
In 200 (16.2%) of the 1236 patients included in either Phase II or Phase III, a MACE occurred within 45 days. Differences between patients with and without MACE are summarized in Table 4. Patients with a MACE within 45 days were older, more often male, and had more often diabetes or hypercholesterolaemia. Occurrence of 45 days MACE was 16.6% in Phase II and 15.7% in Phase III (P = 0.67). The absolute difference in MACE was therefore 0.9% (95% CI −5.0% to 3.2%). The upper limit of 3.2% is under 7.5% meaning non-inferiority can be declared.
Table 4.
Comparison of patients with and without MACE within 45 days in 1236 patients with suspected ACS who had pre-hospital risk assessment
| MACE | No MACE | P-value | |
|---|---|---|---|
| n = 200 | n = 1036 | ||
| Demographics | |||
| Mean age, years (SD) | 68.7 (11.8) | 62.2 (14.3) | 0.01 |
| Male, n (%) | 152 (76) | 559 (54) | 0.01 |
| HEART > 3 | 188 (94) | 674 (65) | 0.01 |
| Cardiac risk factors, n (%) | |||
| Diabetes mellitus | 46 (23) | 148 (14.6) | 0.01 |
| Obesity (body mass index ≥30 kg/m2) | 38 (20) | 164 (18) | 0.50 |
| Hypercholesterolaemia | 89 (45) | 318 (31) | 0.01 |
| Hypertension | 102 (51) | 500 (49) | 0.52 |
| Positive family history | 73 (37) | 439 (43) | 0.10 |
| Current smoking | 44 (22) | 229 (23) | 0.95 |
HEART, History, ECG, Age, Risk factors, and initial Troponin; MACE, major adverse cardiac event.
Percentage MACE in low-risk patients was 2.9% in Phase II and 1.3% in Phase III. In those patients, the absolute difference in MACE was 1.6% (95% CI −1.5% to 4.7%). Table 5 summarizes the differences between the low-risk patients in Phases II and III, including the differences between transferred and not transferred low-risk patients in Phase III.
Table 5.
Differences between patients with low risk (HEART score ≤ 3) in Phases II and III
| Phase II | Phase III | Phase III | P-valuea | |
|---|---|---|---|---|
| Not transferred | Transferred | |||
| n = 172 | n = 149 | n = 53 | ||
| Demographics | ||||
| Mean age, years (SD) | 53.9 (12.2) | 53.8 (14.6) | 54.4 (14.3) | 0.65 |
| Male, n (%) | 86 (50.0) | 75 (50.3) | 32 (60.4) | 0.21 |
| Cardiac risk factors, n (%) | ||||
| Diabetes mellitus | 9 (5.3) | 8 (5.4) | 0 (0) | 0.085 |
| Obesity (body mass index ≥ 30 kg/m2) | 33 (27.7) | 14 (9.4) | 5 (9.4) | 0.99 |
| Hypercholesterolaemia | 46 (27.4) | 20 (13.4) | 8 (15.1) | 0.76 |
| Hypertension | 62 (36.5) | 69 (46.3) | 24 (45.3) | 0.90 |
| Positive family history | 77 (45.8) | 49 (32.9) | 18 (34.0) | 0.89 |
| Current smoking | 44 (26.2) | 39 (26.2) | 15 (28) | 0.76 |
| ‘Gut feeling’ paramedic/consultation cardiologist | 18 (34) | |||
| (1 MACE) | ||||
| Referred by GP | 17 (32.1) | |||
| (0 MACE) | ||||
| Other | 3 (5.7) | |||
| (0 MACE) | ||||
| After 2nd ambulance visit | 17 (32.1) | |||
| (4 MACE) | ||||
| MACE, n (%) | 5 (2.9) | 2 (1.3) | 5 (9.4) | 0.006 |
| ACS | 4 (2.3) | 1 (0.7) | 3 (5.7) | 0.025 |
| PCI | 4 (2.3) | 1 (0.7) | 5 (9.4) | 0.001 |
| CABG | 0 (0) | 0 (0.0) | 1 (1.9) | 0.093 |
| Death (%) | 0 (0) | 1 (0.7) | 0 (0) | 0.55 |
ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; GP, general practitioner; MACE, major adverse cardiac event; PCI, percutaneous coronary intervention.
P-value transferred vs. not transferred in patients with low risk (HEART score ≤ 3) in Phase III.
Of the patients included in FamouS Triage III, a total of three patients died within 45 days. Two of them were transferred to the hospital (HEART score 8 and 9), one patient was not transferred to the hospital (HEART score 3) and died 5 days after inclusion while under treatment by the general practitioner on suspicion of pneumonia.
No MACE endpoint occurred in the 14 patients who were not transferred to the hospital with a HEART score of >3.
Differences in clinical endpoints in the second and third phase of FamouS Triage are summarized in Table 6. The incidence of the primary outcome, MACE within 45 days, was 16.6% in Phase II and 15.7% in Phase III (P = 0.67). Univariate Cox regression analysis resulted in an HR of 0.94 (95% CI 0.69–1.27) to have a MACE within 45 days in Phase III vs. Phase II. Multivariate Cox regression including age and gender, resulted in an HR of 0.93 (95% CI 0.68–1.27). Multivariate Cox regression including age, gender, body mass index, hypercholesterolaemia, and hypertension, resulted in an HR of 0.88 (95% CI 0.63–1.25) for MACE in Phase III vs. Phase II.
Table 6.
Clinical endpoints within 45 days in patients included in either the second phase (FT2) or third phase (FT3)
| Characteristics | FT2 | FT3 | P-value |
|---|---|---|---|
| n = 700 (%) | n = 536 (%) | ||
| MACE, n (%) | 116 (16.6) | 84 (15.7) | 0.67 |
| ACS | 100 (14.3) | 73 (13.6) | 0.74 |
| PCI | 70 (10.0) | 56 (10.4) | 0.80 |
| CABG | 24.3 (3.4) | 15 (2.8) | 0.53 |
| Death | 6 (0.9) | 3 (0.6) | 0.54 |
ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; MACE, major adverse cardiac event; PCI, percutaneous coronary intervention.
As part of the secondary endpoints, follow-up was also conducted after 6 months, this revealed equal results.
Discussion
FamouS Triage is the first study in which a complete HEART score was implemented in the pre-hospital referral decision making. The study confirmed that pre-hospital triage using the HEART score and leaving low-risk patients at home seems non-inferior to standard care in which all patients are transferred to the hospital.
NSTE-ACS is a potentially life-threatening condition in which early risk assessment is essential. Currently, several studies, demonstrated that in patients with suspected NSTE-ACS pre-hospital risk assessment is feasible, and that outcome is comparable with risk assessments performed in the hospital.30,31 Although previous studies showed promising results, structured pre-hospital risk assessment is not yet implemented in routine daily practice.
Although 28% of patients in Phase III were not transferred to the hospital, a substantial part of patients were still transferred to the hospital despite a low-risk classification. Most of these patients (18) were transported based on a, according to the paramedics, suspicious anamnesis for either a cardiac or other serious diagnosis and/or after telephonic consultation with a cardiologist in the hospital.
Seventeen patients already were referred by their general practitioner but besides that included in the study. No MACE endpoints occurred in the low-risk patient group referred by the general practitioners. In the Netherlands, in general, general practitioners neither have the ability to take an ECG, nor the possibility to measure troponin, nor use a validated risk stratification tool in deciding whether or not to refer a patient.
Also one patient with a low HEART score was transported to the hospital based on a positive POC troponin measurement and turned out to have a pulmonary embolism.
Seventeen patients were transferred to the hospital after the second ambulance visit. In 12 of the 17 cases a higher HEART score was observed due to, for example, a dynamic ECG or an increase in complaints. In the other five patients, another diagnosis was suspected at the second visit for which analysis seemed justified.
In our study, patients with complaints suspicious for NSTE-ACS with a low HEART score were not transported to a hospital which may mean that current guidelines sometimes are not followed. For example, current guidelines recommend rhythm monitoring up to 24 h or to PCI (whichever comes first) in confirmed NSTEMI patients at low risk for cardiac arrhythmias (I C recommendation). However, since we visited low-risk patient two times and a second complete HEART score is performed, the risk of missing an NSTEMI is very low. Patients visited by an ambulance because of suspected NSTE-ACS that are at low risk have most likely benign chest pain. The most common non-cardiac causes of the complaints include gastrointestinal or musculoskeletal disorders, pulmonary embolism, panic disorder and anxiety.32 In some patients, urgent diagnostic tests may be mandatory, to exclude other potentially dangerous diagnoses such as pulmonary embolism. The decision to perform other diagnostic tests or to transfer a patient to the ED can be made by either the ambulance paramedic or the general practitioner and may be particularly of importance in case of persisting complaints. Patients with no recurrence of symptoms and none of the very high or high-risk criteria listed in the recommendation table regarding timing of invasive strategy are to be considered at low risk of short-term acute ischaemic events. A selective invasive strategy after appropriate ischaemia testing or detection of obstructive coronary artery disease by coronary computed tomography (CT) angiography is recommended in patients considered at low risk. Out-patient ischaemia testing can be examined by either a cardiologist or a general practitioner, dependent on the local situation and protocols. In our area, general practitioners have the opportunity to refer patients for exercise testing or even coronary CT angiography.
Any subsequent examination via that route may then still lead to a coronary angiography and possibly an elective intervention (and thus a MACE). The question is whether this is undesirable or actually even a better routing of necessary care.
Strengths and limitations
Our study has several strengths. First, it is the first prospective non-inferiority study on referral decisions based on pre-hospital risk assessment in suspected NSTE-ACS. Since patients in the area of two large hospitals, covering rural as well as more densely populated urban areas of the Netherlands, were included, the results are well generalizable.
Our study also has several limitations. We did not randomize patients, but used historical controls. As was demonstrated, there were some significant differences in baseline characteristics between the two phases of the study. In Phase III, a lower risk population was included, possibly because in the first period of Phase III, higher-risk patients were not included (but directly transferred to the hospital). But also patients who in the normal daily practice were already left at home (without using the HEART score) were now included in the study. We adjusted for the known potential confounders in the analyses, and demonstrated that also after multivariate analyses there was no increased risk of 45 days MACE in Phase III. However, in this design of study, there is always a risk that unidentified factors cause selection bias also considering the possible learning curve of paramedics that participated in Phases II and III. We do think the HEART score is a clear and easy to determine tool what should limit the effect of a learning curve, but still this cannot be excluded. Furthermore, our sample size was too small to perform sub-analyses, for example in age groups or male vs. female. Moreover, we are not certain whether our results can be extrapolated to other countries. In the Netherlands, ambulance paramedics have bachelor degrees in nursing with at least two subsequent specializations in critical care nursing.30,33 Furthermore, the paramedics in our study were familiar with the HEART score since 2012 and they were additionally trained in assessing the HEART score including troponin assessment before start of this phase of the FamouS Triage project. Despite this, a substantial proportion of low-risk patients were still referred to the hospital. In addition to the HEART score, which is an important tool, the professional judgement and ‘gut feeling’ of the paramedic may be obviously still important.
It is important to state that the HEART score will be helpful to paramedics, but the score itself is not leading. Of course, it remains important to take into account differential diagnoses indicating other diagnoses that require further analysis in the hospital. However, it is expected that using a validated risk stratification instrument, more patients will not need to be referred than is currently occurring based solely on clinical judgement/feeling.
The phenomenon that the recommendation of the score is not always adhered to was also seen in clinical studies by Poldervaart et al.2 Possible explanations given for this are also valid in the pre-clinical setting. A possible explanation for non-adherence can be the difficulty in changing behaviour. Getting used to calculations and adapting to new algorithms takes time. If the approach is implemented for a longer period and more low-risk patients are not transported without compromising safety, confidence in this new approach is likely to increase.
All patients who were not referred were instructed to contact the dispatch centre again in case of new or worsening symptoms. Unfortunately, it was not registered whether the paramedics had also not referred a patient without the knowledge that a second check-up visit would follow. This monitoring visit proved to be a safety net on more than one occasion.
In order to comply with the current NSTEMI guideline, which recommends a second troponin measurement when complaints started within 6 h, a second HEART score including troponin measurement was performed in our study. However, the original HEART score validation studies did not assess a second troponin measurement.16,34 Although the currently used POC troponin measurement is deemed sufficient for pre-hospital risk stratification with the HEART score,35 a high-sensitive POC measurement can even better risk stratify patients. Because of the lesser sensitivity of the current POC assay, our findings may overestimate the number of patients with a HEART score of ≤3 compared with what would be observed with a high-sensitivity assay. The development of high sensitive POC troponin measurement may result in a modified T-score within the total HEART score, based on new and safer cut-off values. Especially if the under reference limit of troponin detection of these devices is below the 99th percentile. This will further improve quality and safety of referral decisions. Apart from that, new insights are emerging that single troponin tests instead of serial testing in managing patients suspected for NSTE-ACS appears to be safe and is a reasonable strategy to possibly improve efficiency without an adverse association with patient outcomes.36
Future implications
Through early identification of patients with suspected NSTE-ACS who in fact need neither (cardiology) admission nor hospital diagnostics, unnecessary transfer to a hospital can be avoided. This can reduce healthcare expenditures, which is an important focus for improvement of the current healthcare system.37–39 Another point of interest may be pre-hospital identification of high-risk patients in need of acute revascularization which can lead to direct transfer to an interventional centre with subsequent reduction and reduction of costly interhospital transfers.19
Future studies should explore the importance of the added value of the clinical judgement of paramedics in deciding to refer a low-risk patient and whether the occurrence of MACE is still low if more low-risk patients are not transferred to the hospital when following risk stratification more consistently.
Finally, the expectation is that risk stratification can improve even further as soon as it is possible to determine POC high-sensitive troponin. This, in combination with the use of a validated risk stratification tool such as the HEART score, could then function even for a single medical assessment by a paramedic.
Summary
The FamouS Triage study showed that pre-hospital risk stratification of patients with suspected NSTE-ACS, with not transferring low-risk patients to a hospital, seems feasible and non-inferior to transferring all patients to the hospital. If this observation is confirmed by other studies, ambulance guidelines can be adapted with considerable decrease of ED presentations.
Acknowledgements
In the first place, the authors would like to thank the ambulance care professionals for their contribution to the study. Without their professional commitment, such pre-hospital research would not be possible. In addition, a word of appreciation is addressed to the colleagues of the Clinical Chemistry Laboratories, Isala Academy, and the cardiology research office of the Deventer hospital for their assisting contributions to the study.
Funding
The study was investigator driven and was supported by an Isala Research Fun (INNO1326). Roche Diagnostics provided POC devices and POC strips. The researchers were independent of funders and funders were not involved in writing this research or performing analyses.
Conflict of interest: All authors declare to have no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. All authors are responsible for the design and conduct of this study, all study analyses and drafting and editing of the manuscript.
Data availability statement
RTT and MJF had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are available from the corresponding author, RTT, upon reasonable request. This study was externally and independently monitored, according to ICH-GCP guidelines.
References
- 1. Nawar EW, Niska RW, Xu J.. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data 2007;386:1–32. [PubMed] [Google Scholar]
- 2. Poldervaart JM, Reitsma JB, Backus BE, Koffijberg H, Veldkamp RF, Ten Haaf ME, Appelman Y, Mannaerts HFJ, van Dantzig J-M, van den Heuvel M, El Farissi M, Rensing BJWM, Ernst NMSKJ, Dekker IMC, den Hartog FR, Oosterhof T, Lagerweij GR, Buijs EM, van Hessen MWJ, Landman MAJ, van Kimmenade RRJ, Cozijnsen L, Bucx JJJ, van Ofwegen-Hanekamp CEE, Cramer M-J, Six AJ, Doevendans PA, Hoes AW.. Effect of using the HEART score in patients with chest pain in the emergency department: a stepped-wedge, cluster randomized trial. Ann Intern Med 2017;166:689–697. [DOI] [PubMed] [Google Scholar]
- 3. Hyams JM, Streitz MJ, Oliver JJ, Wood RM, Maksimenko YM, Long B, Barnwell RM, April MD.. Impact of the HEART pathway on admission rates for emergency department patients with chest pain: an external clinical validation Study. J Emerg Med 2018;54:549–557. [DOI] [PubMed] [Google Scholar]
- 4. Cotterill PG, Deb P, Shrank WH, Pines JM.. Variation in chest pain emergency department admission rates and acute myocardial infarction and death within 30 days in the medicare population. Acad Emerg Med 2015;22:955–964. [DOI] [PubMed] [Google Scholar]
- 5. Foy AJ, Liu G, Davidson WR, Sciamanna C, Leslie DL.. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med 2015;175:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Safavi KC, Li S-X, Dharmarajan K, Venkatesh AK, Strait KM, Lin H, Lowe TJ, Fazel R, Nallamothu BK, Krumholz HM.. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med 2014;174:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riley RF, Miller CD, Russell GB, Harper EN, Hiestand BC, Hoekstra JW, Lefebvre CW, Nicks BA, Cline DM, Askew KL, Mahler SA.. Cost analysis of the History, ECG, Age, Risk factors, and initial Troponin (HEART) Pathway randomized control trial. Am J Emerg Med 2017;35:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinstock MB, Weingart S, Orth F, VanFossen D, Kaide C, Anderson J, Newman DH.. Risk for clinically relevant adverse cardiac events in patients with chest pain at hospital admission. JAMA Intern Med 2015;175:1207–1212. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen MB, Stengaard C, Sørensen JT, Riddervold IS, Hansen TM, Giebner M, Rasmussen C-H, Bøtker HE, Terkelsen CJ.. Predictive value of routine point-of-care cardiac troponin T measurement for prehospital diagnosis and risk-stratification in patients with suspected acute myocardial infarction. Eur Hear J Acute Cardiovasc Care 2019;8:299–308. [DOI] [PubMed] [Google Scholar]
- 10. Ishak M, Ali D, Fokkert MJ, Slingerland RJ, Tolsma RT, Badings E, van der Sluis A, van Eenennaam F, Mosterd A, Ten Berg JM, van 't Hof AW.. Fast assessment and management of chest pain patients without ST-elevation in the pre-hospital gateway (FamouS Triage): ruling out a myocardial infarction at home with the modified HEART score. Eur Hear J Acute Cardiovasc Care 2018;7:102–110. [DOI] [PubMed] [Google Scholar]
- 11. Stengaard C, Sørensen JT, Ladefoged SA, Lassen JF, Rasmussen MB, Pedersen CK, Ayer A, Bøtker HE, Terkelsen CJ, Thygesen K.. The potential of optimizing prehospital triage of patients with suspected acute myocardial infarction using high-sensitivity cardiac troponin T and copeptin. Biomarkers 2017;22:351–360. [DOI] [PubMed] [Google Scholar]
- 12. Sørensen JT, Terkelsen CJ, Steengaard C, Lassen JF, Trautner S, Christensen EF, Nielsen TT, Bøtker HE, Andersen HR, Thygesen K.. Prehospital troponin T testing in the diagnosis and triage of patients with suspected acute myocardial infarction. Am J Cardiol 2011;107:1436–1440. [DOI] [PubMed] [Google Scholar]
- 13. Tolsma RT, Van Dongen DN, Fokkert MJ, Ottervanger JP, Van Der Sluis A, Slingerland RJ, Van ’T, Hof AW.. 48 The pre-hospital HEART score is a strong predictor of MACE in patients with suspected non-STEMI. Eur Heart J 2017;38:ehx501.48. [Google Scholar]
- 14. Stopyra JP, Harper WS, Higgins TJ, Prokesova JV, Winslow JE, Nelson RD, Alson RL, Davis CA, Russell GB, Miller CD, Mahler SA.. Prehospital modified HEART score predictive of 30-day adverse cardiac events. Prehosp Disaster Med 2018;33:58–62. [DOI] [PubMed] [Google Scholar]
- 15. Stengaard C, Sørensen JT, Ladefoged SA, Christensen EF, Lassen JF, Bøtker HE, Terkelsen CJ, Thygesen K.. Quantitative point-of-care troponin T measurement for diagnosis and prognosis in patients with a suspected acute myocardial infarction. Am J Cardiol 2013;112:1361–1366. [DOI] [PubMed] [Google Scholar]
- 16. Backus BE, Six AJ, Kelder JC, Bosschaert MAR, Mast EG, Mosterd A, Veldkamp RF, Wardeh AJ, Tio R, Braam R, Monnink SHJ, van Tooren R, Mast TP, van den Akker F, Cramer MJM, Poldervaart JM, Hoes AW, Doevendans PA.. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol 2013;168:2153–2158. [DOI] [PubMed] [Google Scholar]
- 17. van Dongen DN, Tolsma RT, Fokkert MJ, Badings EA, van der Sluis A, Slingerland RJ, van 't Hof AW, Ottervanger JP.. Pre-hospital risk assessment in suspected non-ST-elevation acute coronary syndrome: a prospective observational study. Eur Hear J Acute Cardiovasc Care 2020;9:5–12. [DOI] [PubMed] [Google Scholar]
- 18. van Dongen DN, Tolsma RT, Fokkert MJ, Badings EA, van der Sluis A, Slingerland RJ, van 't Hof AW, van 't Riet E, Ottervanger JP.. Referral decisions based on a prehospital HEART score in suspected non-ST-elevation acute coronary syndrome: design of the FamouS Triage 3 study. Future Cardiol 2020;16:217–226. [DOI] [PubMed] [Google Scholar]
- 19. Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 20. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119–177.28886621 [Google Scholar]
- 21. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ.. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary. J Am Coll Cardiol 2014;64:2645–2687. [DOI] [PubMed] [Google Scholar]
- 22. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand J-P, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon J-L, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035.22923432 [Google Scholar]
- 23. Aujesky D, Roy P-M, Verschuren F, Righini M, Osterwalder J, Egloff M, Renaud B, Verhamme P, Stone RA, Legall C, Sanchez O, Pugh NA, N'gako A, Cornuz J, Hugli O, Beer H-J, Perrier A, Fine MJ, Yealy DM.. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378:41–48. [DOI] [PubMed] [Google Scholar]
- 24. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice M-C, di Mario C, Davies S, van Geuns R-J, Eerdmans P, van Es G-A, Meier B, Jüni P.. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 2008;372:1163–1173. [DOI] [PubMed] [Google Scholar]
- 25. von Birgelen C, Sen H, Lam MK, Danse PW, Jessurun GAJ, Hautvast RWM, van Houwelingen GK, Schramm AR, Gin RMTJ, Louwerenburg JW, de Man FHAF, Stoel MG, Löwik MM, Linssen GCM, Saïd SAM, Nienhuis MB, Verhorst PMJ, Basalus MWZ, Doggen CJM, Tandjung K.. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet 2014;383:413–423. [DOI] [PubMed] [Google Scholar]
- 26. Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, Binet D, Daubert J-C.. A randomized trial of long-term remote monitoring of pacemaker recipients (The COMPAS trial). Eur Heart J 2012;33:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. García-Fernández FJ, Osca Asensi J, Romero R, Fernández Lozano I, Larrazabal JM, Martínez Ferrer J, Ortiz R, Pombo M, Tornés FJ, Moradi Kolbolandi M.. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial (RM-ALONE). Eur Heart J 2019;40:1837–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chow SC, Shao J, Wang H.. Sample Size Calculations in Clinical Research, 2nd ed. Florida: Chapman & Hall/CRC Boca Raton; 2008. [Google Scholar]
- 29.Sealed Envelope Ltd. 2012. Power calculator for binary outcome non-inferiority trial. https://www.sealedenvelope.com/power/binary-noninferior/ (10 October 2017).
- 30. Backus BE, Tolsma RT, Boogers MJ.. The new era of chest pain evaluation in the Netherlands. Eur J Emerg Med 2020;27:243–244. [DOI] [PubMed] [Google Scholar]
- 31. Stopyra JP, Snavely AC, Smith LM, Harris RD, Nelson RD, Winslow JE, Alson RL, Pomper GJ, Riley RF, Ashburn NP, Hendley NW, Gaddy J, Woodrum T, Fornage L, Conner D, Alvarez M, Pflum A, Koehler LE, Miller CD, Mahler SA.. Prehospital use of a modified HEART Pathway and point-of-care troponin to predict cardiovascular events. PLoS One 2020;15:e0239460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McConaghy JR, Sharma M, Patel H.. Acute chest pain in adults: outpatient evaluation. Am Fam Physician 2020;102:721–727. [PubMed] [Google Scholar]
- 33. Backus BE, ter Avest E, Gerretsen BM, Viljac A, Tolsma RT.. Organization of prehospital care in the Netherlands:a perspective article. Eur J Emerg Med 2020;27:398–399. [DOI] [PubMed] [Google Scholar]
- 34. Six AJ, Cullen L, Backus BE, Greenslade J, Parsonage W, Aldous S, Doevendans PA, Than M.. The HEART score for the assessment of patients with chest pain in the emergency department. Crit Pathw Cardiol 2013;12:121–126. [DOI] [PubMed] [Google Scholar]
- 35. van Dongen DN, Fokkert MJ, Tolsma RT, van der Sluis A, Slingerland RJ, Badings EA, van 't Hof AWJ, Ottervanger JP.. Accuracy of pre-hospital HEART score risk classification using point of care versus high sensitive troponin in suspected NSTE-ACS. Am J Emerg Med 2020;38:1616–1620. [DOI] [PubMed] [Google Scholar]
- 36. Wassie M, Lee M-S, Sun BC, Wu Y-L, Baecker AS, Redberg RF, Ferencik M, Shen E, Musigdilok V, Sharp AL.. Single vs serial measurements of cardiac troponin level in the evaluation of patients in the emergency department with suspected acute myocardial infarction. JAMA Netw Open 2021;4:e2037930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Meijer C, Wouterse B, Polder J, Koopmanschap M.. The effect of population aging on health expenditure growth: a critical review. Eur J Ageing 2013;10:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bodenheimer T. High and rising health care costs. Part 1: seeking an explanation. Ann Intern Med 2005;142:847–854. [DOI] [PubMed] [Google Scholar]
- 39. Hartwig J. What drives health care expenditure?–Baumol’s model of ‘unbalanced growth’ revisited. J Health Econ 2008;27:603–623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RTT and MJF had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are available from the corresponding author, RTT, upon reasonable request. This study was externally and independently monitored, according to ICH-GCP guidelines.