SUMMARY
Bacillus cereus group species are widespread, Gram-positive, spore-forming environmental bacteria. B. cereus sensu stricto is one of the major causes of food poisoning worldwide. In high-risk individuals, such as preterm neonates, B. cereus infections can cause fatal infections. It is important to note that the phenotypic identification methods commonly used in clinical microbiology laboratories make no distinction between B. cereus sensu stricto and the other members of the group (Bacillus anthracis excluded). As a result, all the invasive infections attributed to B. cereus are not necessarily due to B. cereus sensu stricto but likely to other closely related species of the B. cereus group. Next-generation sequencing (NGS) should be used to characterize the whole genome of the strains belonging to the B. cereus group. This could confirm whether the strains involved in previously reported B. cereus invasive infections preferentially belong to formerly known or emerging individual species. Moreover, infections related to B. cereus group species have probably been overlooked, since their isolation in human bacteriological samples has for a long time been regarded as an environmental contaminant of the cultures. Recent studies have questioned the emergence or reemergence of B. cereus invasive infections in preterm infants. This review reports our current understanding of B. cereus infections in neonates, including taxonomical updates, microbiological characteristics, bacterial identification, clinical features, host-pathogen interactions, environmental sources of contamination, and antimicrobial resistance.
KEYWORDS: Bacillus cereus, preterm neonates, infection, antimicrobial agents, environmental microbiology, pediatric infectious disease
INTRODUCTION
Bacillus cereus group species consist of large, sporulating, Gram-positive, and rod-shaped aerobic or facultative anaerobic bacteria that are widespread in the environment. These bacteria can be isolated from their environmental reservoir: soil, sea sediments and seawater, plants, and carcasses of animals that died after infection with Bacillus anthracis (1–3). These bacterial species are known to have a significant impact on human health, agriculture, or food industry, especially B. anthracis, B. cereus sensu stricto, and Bacillus thuringiensis (2, 4). B. cereus sensu stricto can contaminate food, especially vegetables and starchy food, and is mainly involved in gastrointestinal (GI) infections in humans. B. cereus sensu stricto represents one of the major causes of food poisoning outbreaks in Europe (5). Furthermore, some strains of B. cereus sensu stricto display a high virulence potential and are involved in various invasive and frequently fatal infections, particularly in immunocompromised patients, patients with substance use disorders, postsurgical patients, and preterm neonates. It is important to keep in mind two major points. First, it is difficult to distinguish between B. cereus sensu stricto and the other members of the group with the phenotypic identification methods routinely used in the clinical microbiology laboratory. Therefore, most human infections attributed to B. cereus in the literature should be considered B. cereus group species infections. Second, invasive infections related to B. cereus have probably been overlooked, since the isolation of B. cereus in human bacteriological samples, especially in blood culture bottles, has for a long time been regarded as an environmental contaminant of the cultures (6). Recent studies have suggested the emergence or reemergence of B. cereus invasive infections in preterm neonates, and a recent epidemiological survey found an increase of B. cereus bacteremia incidence in the neonatal intensive care unit (ICU) at the Assistance Publique des Hôpitaux de Paris (APHP) in France (7). In our tertiary care center in Nice, France, we reported the death of two premature neonates in 2013 despite appropriate wide-spectrum antibiotic treatment (8). The increasing number of recent data concerning B. cereus infections in preterm neonates is of major concern in pediatric public health.
This review reports the current knowledge on B. cereus infections in preterm neonates, including a taxonomical update, microbiological characteristics, clinical features, sources of contamination, and antimicrobial resistance. This review also aims to address the question of whether this environmental bacterium might be involved in lethal infections in premature infants because of the existence of hypervirulent strains or because of the immaturity of the neonatal immune system.
TAXONOMY AND PHYLOGENY OF B. CEREUS GROUP
B. cereus sensu stricto belongs to the subdivision of the Bacillus genus. To date, 22 species have been reported in the literature as closely related to B. cereus sensu stricto (Table 1) (9–22). Among these species, only four new species have also been published but not yet validated by the International Committee of Systematic of Prokaryotes (16, 17, 19, 22). Historically, in the early 2000s, only six species had been described: B. anthracis (9), B. cereus sensu stricto (10), B. thuringiensis (12), Bacillus mycoides (11), Bacillus pseudomycoides (14), and Bacillus weihenstephanenis (23). But, B. weihenstephanenis was reclassified as a later heterotypic synonym of B. mycoides (20). These five species have classically been defined based on the presence of species-specific phenotypic and biochemical characteristics and on a similarity value lower than 70% using DNA/DNA hybridization methods. However, B. cereus sensu stricto and B. thuringiensis have been validated as distinct species, although these two species display a similarity value greater than 70%. Historically, the distinction between these two species was made because they display various pathogenic properties and diverse ecological lifestyles due to the presence or absence of plasmid harboring various toxin genes. Virulent strains of B. cereus sensu stricto can cause an emetic type of food poisoning induced by the production of cereulide, a toxin encoded by the ces gene that is located on a pXO1-like plasmid (24, 25). B. thuringiensis has insecticidal properties due to crystal proteins encoded by plasmid-borne cry genes. B. anthracis is the etiologic agent of anthrax. Pathogenic strains of B. anthracis harbor two virulent plasmids, i.e., pXO1 and pXO2 (26). Over the past 10 years, 17 species have been described as species closely related to B. cereus sensu stricto, mostly since 2010 due to the expansion of whole-genome sequencing techniques (13, 15–22).
TABLE 1.
Type strains phylogenetically closely related to B. cereus sensu stricto
| Species and straina | Location | Yr of isolation | Sample typeb | Growth temp range (°C) (20–22) | Reference |
|---|---|---|---|---|---|
| Bacillus anthracis ATCC 14578T | Germany | 1872 | NA | 10–50 | 9 |
| Bacillus cereus ATCC 14579T | England | 1887 | Air | 10–45 | 10 |
| Bacillus mycoides DSM 11821* | Germany | 1886 | NA | 15–40 | 11 |
| Bacillus thuringiensis ATCC 10792T | Germany | 1915 | Flour moth | 10–45 | 12 |
| Bacillus toyonensis BCT-7112T | Japan | 1966 | Probiotic | 10–45 | 13 |
| Bacillus pseudomycoides DSM 12442T | USA | 1995 | Soil isolate | 10–40 | 14 |
| Bacillus cytotoxicus NVH 391-98T | France | 1998 | Vegetable puree | 20–50 | 15 |
| “Bacillus gaemokensis” BL3-6T | South Korea | 2010 | Sediments from the Yellow Sea | 15–40 | 16 |
| “Bacillus manliponensis” BL4-6T | South Korea | 2011 | Sediments from the Yellow Sea | 15–40 | 17 |
| Bacillus wiedmannii DSM 102050T | USA | 2012 | Dairy products | 5–43 | 18 |
| “Bacillus bingmayongensis” FJAT 13831T | China | 2014 | Soil isolate (Emperor Qin’s terra-cotta warriors) | 15–45 | 19 |
| Bacillus luti KCTC 33716T | China | 2017 | Sediments and seawater (Pacific Ocean) | 10–39 | 20 |
| Bacillus mobilis KCTC 33717T | China | 2017 | Sediments and seawater (Pacific Ocean) | 10–39 | 20 |
| Bacillus nitratireducens KCTC 33713T | China | 2017 | Sediments and seawater (Pacific Ocean) | 7–39 | 20 |
| Bacillus pacificus KCTC 33858T | China | 2017 | Sediments and seawater (Pacific Ocean) | 15–45 | 20 |
| Bacillus paramycoides KCYC 33709T | China | 2017 | Sediments and seawater (Pacific Ocean) | 15–39 | 20 |
| Bacillus paranthracis KCTC 33714T | China | 2017 | Sediments and seawater (Pacific Ocean) | 15–45 | 20 |
| Bacillus proteolyticus KCTC 33715T | China | 2017 | Sediments and seawater (Pacific Ocean) | 10–39 | 20 |
| Bacillus tropicus KCTC 33711T | China | 2017 | Sediments and seawater (Pacific Ocean) | 15–45 | 20 |
| Bacillus albus KCTC 33710T | China | 2017 | Sediments and seawater (Pacific Ocean) | 15–40 | 20 |
| Bacillus fungorum KCTC 33949T | China | 2017 | Spent mushroom substrate | 10–45 | 21 |
| “Bacillus clarus” ATCC 21929T | Papua New Guinea | NA | Soil | 15–43 | 22 |
Species names in quotation marks indicates species not yet validated.
NA, not available.
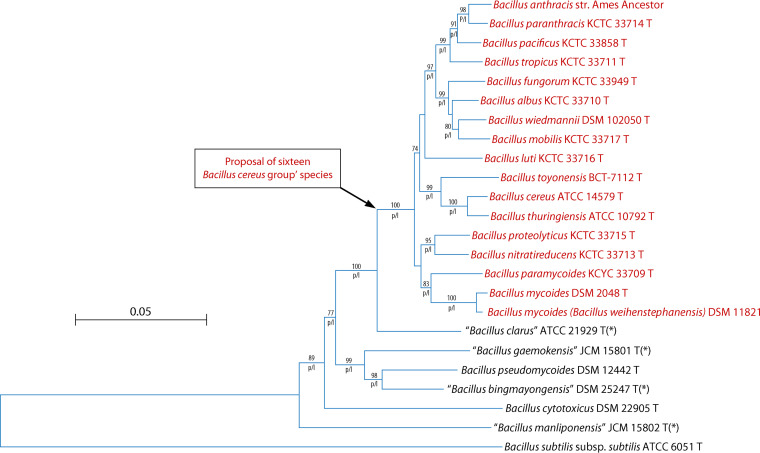
The taxonomic history of the B. cereus group has been recently reviewed (27). However, in the literature, there are no real criteria to confirm that a new species belongs to the B. cereus group. We have studied the phylogenetic relationships between species using multilocus sequence typing (MLST), as described in Fig. 1 (28). The comparison of three phylogenetic methods (neighbor-joining, maximum likelihood, and maximum parsimony), as well as bootstrap replications (×1,000), revealed a solid group consisting of 16 B. cereus species. The members of this group are also distinguished from other species outside the group by a panel of phenotypic characters, as shown in Table 2. Interestingly, the members of this group have mostly been involved in human infections, while species outside this group rarely cause human infections. Furthermore, based on analyses of 2,231 genomes, these 16 species are also in the same group (29). For these four reasons (phylogenetic, phenotypic, and genomic analyses and clinical infection), we could propose to delineate the B. cereus group to those 16 species, as shown in Fig. 1. In the rest of the review, when we refer to “B. cereus,” it will be to the B. cereus group composed by the 16 species.
FIG 1.
Phylogenetic relationships between the species of the Bacillus cereus group with Bacillus subtilis subsp. subtilis used as the outgroup. The tree was obtained by the neighbor-joining method based on a comparison of the concatenated sequences of seven housekeeping genes (glpF, gmk, ilvD, pta, pur, pycA, tpi). The Kimura two-parameter distance measure was used as implemented in MEGA X (28). Values above the lines indicate how the tree’s branches are supported by the results of bootstrap analysis (×100 replicates) (only values greater than 70% are shown). The letters “p” and “l” under the lines indicate branches that were also found by the maximum parsimony method and maximum likelihood method, respectively (28). Scale bar, accumulated changes per nucleotide. Asterisks indicate species published but not yet validated.
TABLE 2.
Main positive biochemical characteristics of type strains for proposed species in B. cereus group and species outside of B. cereus group (20–22)
| Species group | % of species positive for: |
||||||
|---|---|---|---|---|---|---|---|
| Acetoin production | Arbutin | Citrate utilization | Oxidase | Starch hydrolysis | Trehalose | Urease | |
| B. cereus group (n = 16)a | 94 | 82 | 82 | 94 | 78 | 94 | 0 |
| Species outside of B. cereus group (n = 6)b | 60 | 50 | 33 | 50 | 33 | 50 | 33 |
Includes Bacillus paramycoides KCYC 33709T; Bacillus albus KCTC 33710T; Bacillus proteolyticus KCTC 33715T; Bacillus cereus ATCC 14579T; Bacillus anthracis ATCC 14578T; Bacillus paranthracis KCTC 33714T; Bacillus pacificus KCTC 33858T; Bacillus tropicus KCTC 33711T; Bacillus fungorum KCTC 33949T; Bacillus wiedmannii DSM 102050T; Bacillus mobilis KCTC 33717T; Bacillus luti KCTC 33716v; Bacillus thuringiensis ATCC 10792T; Bacillus toyonensis BCT-7112T; Bacillus mycoides DSM 2048T; Bacillus mycoides (weihenstephanensis) DSM 11821; Bacillus nitratireducens KCTC 33713T.
Includes Bacillus pseudomycoides DSM 12442T; Bacillus cytotoxicus NVH 391-98T, “Bacillus bingmayongensis FJAT 13831T,” “Bacillus manliponensis BL4-6T,” “Bacillus gaemokensis JCM 15801T,” and “Bacillus clarus ATCC 21929T.”
Among the new species of the B. cereus group, it is interesting to note that Bacillus paranthracis, initially isolated from sediment of the Pacific Ocean, has recently been implicated in an emetic outbreak (30). In our very recent study, WGS of three strains involved in invasive infections in newborns and belonging to the B. cereus group showed that one of them was B. paranthracis (31). These two recent WGS studies question the role of one individual species, B. paranthracis, in human infections and notably in invasive infections in neonates. These interesting findings need to be further investigated and underline the necessity to develop new identification strategies for discrimination between species.
SPECIES IDENTIFICATION WITHIN THE B. CEREUS GROUP
The bacteria belonging to the B. cereus group are rod-shaped and sporulating Gram-positive bacilli. The members of the B. cereus group, excluding B. anthracis, display various morphological forms depending upon the milieu in which they are observed. In the environment, these bacteria persist as a sporulated and very resistant form. Spores can germinate once in contact with an insect or human host and produce vegetative cells (2, 32). Gram stains of blood culture typically yield straight to slightly curved bacilli with square ends either singly or arranged in pairs or short chains. B. cereus group species are aerobic or facultative anaerobic bacteria and can grow over a broad temperature range (Table 1) (2, 33–35). Colonies are usually dull gray and opaque with a rough surface. When grown at 37°C on enriched blood agar medium under an aerobic atmosphere, strains display various levels of hemolysis, from nonhemolytic to high ß-hemolytic activity (1, 36). Selective culture media for B. cereus group species can also be used in routine microbiology, such as MYPA (mannitol yolk polymyxin B agar), PEMBA (pyruvate egg yolk mannitol blue agar), or the chromogenic medium Brilliance Bacillus cereus agar (BBC) (all ThermoFisher) (37). Several biochemical and phenotypic characteristics of the different species can differentiate the species of the B. cereus group (20). The main differences between positive biochemical characteristics of the type strains for the different proposed species in the B. cereus group and species outside of the B. cereus group are detailed in Table 2 (20–22).
In a routine clinical microbiology laboratory, bacterial identification is now based on the analyses of protein spectra obtained by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), which is a reliable tool for pathogen identification. However, this method still has limitations in identifying closely related microbial species such as the B. cereus group species. To date, the commercial version of the Biotyper database (Bruker Daltonics, Bremen, Germany), which is widely used in routine clinical microbiology laboratories, contains only microbial reference spectra for three species of the B. cereus group, as previously described (see Taxonomy and Phylogeny of B. cereus Group): B. cereus sensu stricto (4 spectra), B. mycoides (3 spectra), and B. thuringiensis (1 spectrum). Some of the newly described species of the B. cereus group are still missing from this database. With respect to the last version of the Vitek MS 3.2 database (bioMérieux, France), it contains reference spectra for B. cereus sensu stricto, B. mycoides, B. thuringiensis, and B. weihenstephanensis. When a bacterial spectrum matches one of these four spectra, the bacterium is identified as “B. cereus group.” Moreover, MALDI-TOF MS could misidentify strains belonging to the B. cereus group. For example, the foodborne human pathogen B. cereus sensu stricto can be misidentified as B. thuringiensis, an insect pathogen widely used as a biopesticide and very closely phylogenetically related to B. cereus sensu stricto. Indeed, these two species differ only by the presence or absence of the Cry toxin-encoding plasmids. B. cereus sensu stricto can also be misidentified as B. anthracis. If these two species are phylogenetically related, B. anthracis can be implicated in anthrax, a life-threatening disease caused by the production of anthrax toxin. To overcome this important limitation in discerning B. cereus group species, some researchers have tried to optimize MALDI-TOF identification methods provided by the manufacturers by enrichment of available spectra and by using specific algorithms allowing discrimination between the different species of the B. cereus group (38–40).
Molecular approaches such as 16S rRNA gene sequencing are widely used for routine bacterial identification (41). However, as suggested by previous studies, 16S rRNA gene sequencing has failed to discriminate between B. cereus, B. thuringiensis, and B. anthracis because of the high similarity in 16S rRNA gene sequences between these three species.
To conclude, we recommend the use of MLST (as described in Taxonomy and Phylogeny of B. cereus Group) and determination of a panel of phenotypic characters (Table 2) in order to classify a species as belonging to the B. cereus group as defined above. Furthermore, promising tools such as WGS using core genome MLST or new MALDI-TOF approaches as described above (38–40) should be performed to reach identification to the species level.
HABITAT AND EPIDEMIOLOGY
B. cereus is widespread in nature. The natural environmental reservoir for B. cereus consists of soil, decomposing organic matter, fresh and marine water, plants, and the intestinal tracts of invertebrates (2, 42). From this natural habitat, the microorganism is able to contaminate a wide variety of food products, and this can lead to the transient colonization of the human gut (43). Moreover, B. cereus is capable of growing quickly under a wide variety of conditions, which explains its ubiquitous worldwide distribution regardless of the environment. B. cereus also produces endospores that enable it to withstand desiccation, temperature and pH variations, and anaerobic conditions (34). Interestingly, the spores of certain B. cereus strains are inactivated by a heat shock treatment of 10 to 20 min at 90°C and 2 min at 95°C. According to Stadhouders et al., a heat shock treatment of 10 to 20 s at 125°C is necessary to inactivate B. cereus spores in milk (44). This ability to survive under unfavorable conditions makes it difficult to eliminate in areas such as food production or hospital-based care services because the spores can adhere to surfaces and are resistant to pasteurization. Some B. cereus strains are also able to form diverse biofilms that allow them to resist biocleaning procedures (45). Biofilm formation and sporulation are therefore responsible for the persistence of B. cereus in the environment. In general, human B. cereus infections occur via ingestion of contaminated food, inhalation of spores, or direct inoculation into the skin.
EXTRADIGESTIVE AND INVASIVE B. CEREUS INFECTIONS
B. cereus is an opportunistic pathogen responsible for extradigestive, localized or systemic, nosocomial infections, frequently occurring in immunocompromised patients and in newborns (premature or full-term). In these populations, B. cereus causes various types of infections, including sepsis, septic shock, central nervous system (CNS) infections, and even eye infections (2). Recently, Messelhäußer and Ehling-Schulz have summarized the main cases of extradigestive infections described in the literature between 2012 and 2017. In this review, they report bacteremia or sepsis for 42% of cases, CNS infections with cerebral damage (abscess, meningoencephalitis) for 21% of cases, endocarditis and eye infections for 17% of cases, two cases of necrotizing fasciitis, and one case of peritonitis and hepatic abscess (34). In immunocompromised subjects, the main risk factors for infection are malignant hemopathies, intravascular devices, intravenous drug injections, and traumatic or surgical lesions (46, 47). In immunocompetent patients, systemic infections such as anthrax-like disease type and catheter-related bloodstream infections (CRBSI) have also been reported (48, 49). In immunocompetent adults, systemic B. cereus infections are rarely fatal, unlike infections that occur in at-risk populations such as preterm neonates, where the fatality rate can reach up to 30%, as shown by Fournier and colleagues (7).
Overall, there is a wide clinical spectrum of human B. cereus infections. Indeed, as already mentioned, extradigestive infections are probably overlooked, because B. cereus has, for a long time, been regarded as an environmental contaminant when isolated in bacteriological samples, especially in blood cultures, due to its wide distribution in the environment. However, the increasing volume of recent data concerning B. cereus invasive and frequently fatal infections in preterm neonates is of major concern in pediatric public health and is discussed hereafter.
EMERGENCE OF B. CEREUS INFECTIONS IN PRETERM NEONATES
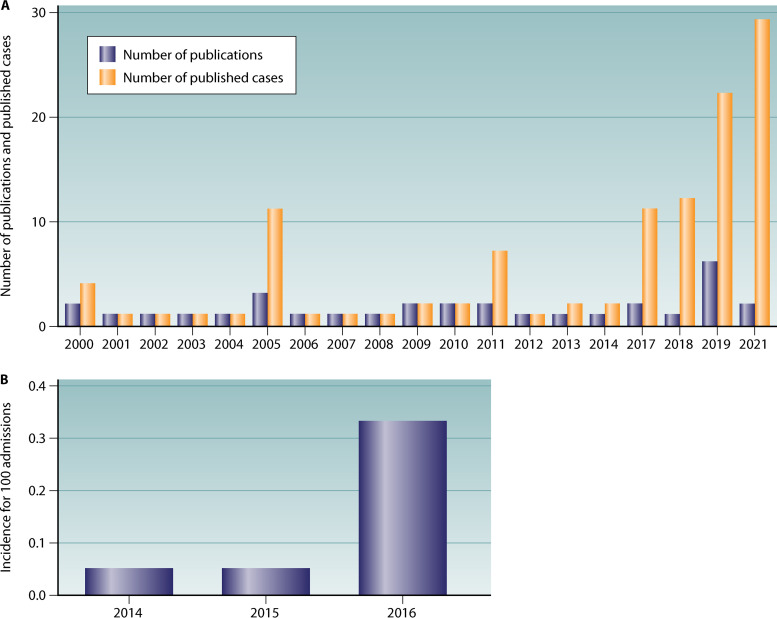
B. cereus has an emerging role in opportunistic infections in at-risk populations such as the elderly, immunocompromised patients, and preterm neonates. In this review, we focus on B. cereus invasive infections in infants, which are being increasingly reported in the literature (Fig. 2). A newborn is considered premature when birth occurs before the start of the 37th week of pregnancy (50). Invasive infections in these patients occur sporadically or in outbreaks. A very recent description of nosocomial outbreaks in France, Germany, and Israel led to a reconsideration of the global risk of this potentially serious burden (7, 51–53). Therefore, improving the current knowledge regarding B. cereus pathogenesis, transmission risks, and treatment is of major concern in pediatric public health. We performed a survey of the literature between January 1977 and January 2021 for data on B. cereus infections in neonates by using Medline and Scopus. The search terms B. cereus, Bacillus cereus, newborn, neonate, infant, premature, preterm, and neonatal were used.
FIG 2.
(A) Evolution of the number of publications and number of published cases of Bacillus cereus infections in preterm neonates per year for 2000 to 2021. (B) Evolution of the incidence of Bacillus cereus bacteremia in preterm neonates (number per 100 admissions) for 2014 to 2016 at Assistance Publique des Hôpitaux de Paris, Paris, France (7).
To date, 145 cases have been reported in 106 patients, including 69 cases of bacteremia (48%) (7, 8, 51–77), 36 CNS infections (25%) (8, 51, 53, 55–57, 61–64, 67, 68, 70, 71, 73–82), 18 respiratory tract infections (12%) (8, 52, 53, 56, 74, 76, 83), 13 cases of skin infections (9%) (53, 84), six cases of GI infections (4%) (53, 85, 86), two cases of osteoarticular infections (1%) (71, 72), and a single case of urinary tract infection (UTI) (1%) (53) (Table 3). B. cereus infection was fatal in 33 of 106 patients (31%). Regarding treatment, the implementation of antibiotic treatment, based on vancomycin for B. cereus invasive infection (87), did not prevent patient death in 11 of 33 cases (33%) when considering only patients for whom antibiotic therapy information was available (51, 52, 60, 70, 72, 75, 79, 88).
TABLE 3.
Type of infection due to B. cereus in neonates
| Type of infection | No. (%) of cases (n = 145) | Source(s) of positive clinical specimensa | Reference(s) (no. of cases) |
|---|---|---|---|
| Bacteremia | 69 (48) | Blood culture | 54 (1), 88 (2), 51 (3), 52 (1), 55 (1), 53 (6), 7, (9), 8 (1), 56 (2), 57 (1), 58 (3), 59 (8), 60 (2), 61 (1), 62 (1), 63 (1), 64 (1), 65 (1), 66 (8), 67 (3), 68 (1), 69 (1), 70 (1), 71 (3), 72 (1), 73 (1), 74 (2), 75 (1), 77 (1), 76 (1) |
| CNS infection | 36 (25) | ||
| Meningitis, meningoencephalitis | 23 (16) | CSF, meninges, brain tissue | 53 (1), 57 (1), 61 (1), 62 (1), 64 (1), 67 (3), 68 (1), 70 (1), 71 (2), 73 (2), 74 (1), 75 (1), 77 (1), 78 (1), 79 (1), 80 (2), 81 (1), 82 (1) |
| Brain abscesses, empyema, or necrosis | 13 (9) | Brain tissue, necrosis | 51 (1), 55 (1), 53 (1, 1), 8 (1), 56 (1), 62 (1), 63 (1), 64 (1), 68 (1), 70 (1), 75 (1), 74 (1), 76 (1) |
| Respiratory infection | 18 (12) | ||
| Pneumonia | 11 (8) | Tracheal aspiration, pleural fluid, lung tissue | 52 (2), 53 (1), 74 (2), 89 (1), 8 (1), 83 (4) |
| Pulmonary abscesses or necrosis | 6 (4) | Lung tissue, pleural fluid | 52 (1), 56 (1), 74 (2), 76 (1), 89 (1) |
| Tracheobronchitis | 1 (0.7) | NA | 52 (1) |
| Cutaneous infection | 13 (9) | Skin, armpit, umbilical cord stump swab | 84 (12), 53 (1) |
| Gastrointestinal infection | 6 (4) | ||
| Digestive tract | 5 (3.5) | Gastric fluid, stomach tube feeding, sample from abdominal cavity | 53 (2), 85 (2), 86 (1) |
| Liver | 1 (0.7) | NA | 53 (1) |
| Osteoarticular infection | 2 (1) | ||
| Arthritis | 1 (0.7) | Synovial fluid | 71 (1) |
| Osteitis | 1 (0.7) | Bone, bone marrow | 72 (1) |
| Kidney and urinary infection | 1 (1) | NA | 53 (1) |
CSF, cerebrospinal fluid; NA, not available.
Collectively, two groups of infections stand out from the data found in the literature regarding B. cereus infections in newborns: systemic involvement with bacteremia and other serious infections without bacteremia.
Invasive Infections with B. cereus Bacteremia
We further analyzed a total of 69 cases of B. cereus bacteremia in newborns (7, 8, 51–77, 88) (Table 4). The selection criterion was the presence of at least one positive blood culture for B. cereus in a patient. All cases have been described in neonatal or neonatal ICU departments. Eighty-one percent of patients were premature newborns, while 19% were born after the 37th week of gestation. The mean gestational age at birth was 30 weeks, and the mean weight of birth was 1,392 g. The presence of B. cereus in the blood culture was considered to be contamination in only four cases (4/69, 6%) (51, 59). Indeed, in these four cases, B. cereus was found in blood culture bottles (BCBs), and the neonate did not have any sign of infection. Therefore, the presence of B. cereus in the BCBs was considered a contamination of the samples from the environmental reservoir of B. cereus. In all other reports, B. cereus was responsible for sepsis (50/69, 72%) (7, 51, 53–57, 59, 60, 62, 65–68, 70–73, 76, 77, 82) or septic shock (15/69, 22%) (8, 51, 52, 56, 60, 61, 64, 69, 71, 74, 75, 88). A peculiarity of B. cereus sepsis in neonates is the secondary meningeal or cerebral dissemination. Indeed, meningitis or meningoencephalitis (53, 57, 61, 62, 64, 67, 70, 71, 73–75, 77) and brain abscesses or necrosis (7, 8, 51, 53, 55, 56, 62–64, 68, 75, 76) were reported in 19/65 (29%) and 12/65 (18%) of the neonates presenting with B. cereus-related sepsis and septic shock, respectively. Pneumonia was associated in 6 of 65 patients (9%) (52, 53, 56, 74, 76).
TABLE 4.
Main features of reported cases of B. cereus bacteremia in neonatesa
| Reference (n, no. of cases) | Term of birth (wks + days of gestation) | Wt at birth (g) | Underlying condition(s) | Clinical presentation | Disseminated infection | Suspected source(s) of Infection | Antibiotic treatmentb | Outcome |
|---|---|---|---|---|---|---|---|---|
| Liao and Tsai, 2021 (54) (n = 1) | 33 + 2 | 1490 | Respiratory distress | Sepsis | No | Mother’s breast milk | VCM, AMK | Recovery |
| Lewin et al., 2019 (88) (n = 2) | 25 | 590 | Hyaline membrane disease, pulmonary hypertension | Septic shock | No | Banked human milk | LNZ, MRP, VCM | Death |
| 24 | 560 | Chorioamnionitis, CBD, patent ductus arterioses | Septic shock | No | Banked human milk | AMP, TBM, VCM, MRP, PTZ, CTX | Death | |
| Bar-Meir et al., 2019 (51) (n = 3) | 32 | NA | Twin pregnancy | Septic shock | Brain abscesses | Construction-related dust | VCM, MRP, CRP | Death |
| 31 | NA | No | Sepsis | No | Construction-related dust | VCM, MRP | Recovery | |
| 31 | NA | CBD | Contamination | No | Construction-related dust | None | Recovery | |
| Papan et al., 2019 (52) (n = 1) | 37 + 5 | 2,700 | Congenital diaphragmatic hernia closure | Septic shock | Pneumonia, pyogenic tracheobronchitis, necrotizing bronchiolitis | Undetermined | VCM, MRP, FSF | Death |
| Samarasekara et al., 2020 (55) (n = 1) | 30 | NA | No | Sepsis | Brain abscess | Undetermined | VCM, MRP, AMP, FCX | Recovery |
| Glasset et al., 2018 (53) (n = 6) | NA | NA | NA | Sepsis | Meningitis, pulmonary and liver infection | Surface in medical ward | None | Death |
| NA | NA | NA | Sepsis | Brain abscess | Surface in medical ward | VCM, CFT | Recovery | |
| NA | NA | NA | Sepsis | No | Surface in medical ward | VCM | Recovery | |
| NA | NA | NA | Sepsis | No | Surface in medical ward | VCM, AMK, AMX | Recovery | |
| NA | NA | NA | Sepsis | No | Surface in medical ward, central catheter | VCM | Recovery | |
| NA | NA | NA | Sepsis | Kidney and urinary infection | Undetermined | CFX, GTM | Recovery | |
| Fournier et al., 2017 (7) (n = 9) | 30 + 2 | 750 | Intrauterine growth restriction, hemorrhagic brain lesions | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Death |
| 29 + 2 | 1,075 | Twin pregnancy, transfused transfusion syndrome | Sepsis | Extensive brain damage | Banked human milk, parenteral nutritional solutions | NA | Death | |
| 37 + 2 | 2,815 | Atresia of the small intestine | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Recovery | |
| 31 | 1,380 | Atresia of the duodenum | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Recovery | |
| 29 + 4 | 1,025 | Umbilical hernia | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Recovery | |
| 27 + 5 | 750 | Enterocolitis | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Death | |
| 31 | 1,720 | Twin pregnancy | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Recovery | |
| 38 + 6 | 3,515 | Neonatal respiratory distress | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Recovery | |
| 39 | 3,240 | Polymalformation | Sepsis | No | Banked human milk, parenteral nutritional solutions | NA | Recovery | |
| Lotte et al., 2017 (8) (n = 1) | 29 + 4 | 1,480 | Maternal malignancy | Septic shock | Brain empyema, abscesses and necrosis, cranial hemorrhages | Central catheter, surface in medical ward | VCM, GTM, CFT | Death |
| Ramarao et al., 2014 (56) (n = 2) | 24 + 5 | 650 | CBD | Sepsis | No | Central catheter | VCM, AMK, CFT | Recovery |
| 26 + 5 | 615 | No | Septic shock | Brain and pulmonary abscesses | Central catheter | None | Death | |
| Horii et al., 2012 (57) (n = 1) | NA | 800 | Bowel perforation, CBD | Sepsis | Late meningitis | Hospital linens | VCM, MRP, LNZ, CLD | Recovery |
| Shimono et al. 2012, (58) (n = 3) | 29 | 476 | NA | Sepsis | No | Surface in medical ward | NA | Recovery |
| 30 | 876 | NA | Sepsis | No | Surface in medical ward | NA | Recovery | |
| 28 | 1,018 | NA | Sepsis | No | Surface in medical ward | NA | Recovery | |
| Campbell et al., 2011 (59) (n = 8) | 31 | 1,650 | NA | Sepsis | No | Endotracheal intubation, central catheter, construction excavation | VCM | Recovery |
| 29 | 1,148 | NA | Sepsis | No | Endotracheal intubation, central catheter, construction excavation | VCM | Recovery | |
| 28 | 1,515 | NA | Sepsis | No | Endotracheal intubation, central catheter, construction excavation | VCM | Recovery | |
| 24 | 710 | NA | Sepsis | No | Endotracheal intubation, central catheter, construction excavation | VCM | Recovery | |
| 25 | 945 | NA | Sepsis | No | Endotracheal intubation, central catheter, construction excavation | VCM | Recovery | |
| 29 | 1,015 | NA | Contamination | No | Endotracheal intubation, central catheter, construction excavation | None | Recovery | |
| 40 | 4,184 | NA | Contamination | No | Endotracheal intubation, central catheter, construction excavation | None | Recovery | |
| 30 | 870 | NA | Contamination | No | Endotracheal intubation, central catheter, construction excavation | None | Recovery | |
| Sasahara et al., 2011 (60) (n = 2) | NA | NA | Mitral regurgitation | Septic shock | No | Hospital linens, central catheter, parenteral solutions | AMP/SBT, MRP, VCM, PNP | Death |
| NA | NA | Patent ductus arteriosus | Septic Shock | No | Hospital linens, central catheter, parenteral solutions | CFZ | Death | |
| Saito et al., 2010 (61) (n = 1) | 27 | 740 | Intrauterine growth restriction, patent ductus arteriosus, severe pregnancy-induced hypertension | Septic shock | Meningitis, intraventricular hemorrhage, pulmonary hemorrhage | Undetermined | CFZ | Death |
| Drazin et al., 2010 (62) (n = 1) | 32 + 4 | 1,910 | Mild dysmorphic features, patent foramen ovale, twin pregnancy, preeclampsia | Sepsis | Meningoencephalitis, brain abscesses | Undetermined | VCM, AMK, GTM, MRP | Recovery |
| Pawlik et al., 2009 (63) (n = 1) | 27 | 730 | NA | Sepsis | Brain abscesses | Undetermined | NA | Recovery |
| Evreux et al., 2007 (64) (n = 1) | 31 | 1,670 | Thyroid agenesis | Septic shock | Meningitis, brain abscesses and necrosis | Central catheter | CFT, AMX, MTZ, AMK | Death |
| John et al., 2006 (65) (n = 1) | 32 | 1,512 | Ruptured bicornuate uterus, severe fetal distress | Sepsis | Subependymal hemorrhage, cerebral edema | Undetermined | PTZ, AMK, VCM | Recovery |
| Adler et al., 2005 (66) (n = 8) | 25 | 680 | Candida sepsis, chronic lung disease, retinopathy | Sepsis | No | Air | MRP | Recovery |
| 34 | 2,010 | Escherichia coli peritonitis | Sepsis | No | Air | MRP | Recovery | |
| 37 | 2,000 | Klebsiella peritonitis and sepsis | Sepsis | No | Air | VCM | Recovery | |
| 36 | 1,735 | Necrotizing enterocolitis | Sepsis | No | Air | MRP | Recovery | |
| 30 | 1,226 | Necrotizing enterocolitis | Sepsis | No | Air | MRP | Recovery | |
| 29 | 1,508 | No | Sepsis | No | Air | VCM | Recovery | |
| 32 | 1600 | No | Sepsis | No | Air | VCM | Recovery | |
| 27 | 870 | No | Sepsis | No | Air | VCM | Recovery | |
| Lequin et al., 2005 (67) (n = 3) | 30 + 6 | NA | No | Sepsis | Hemorrhagic meningoencephalitis | Undetermined | NA | Death |
| 28 + 3 | NA | No | Sepsis | Hemorrhagic meningoencephalitis | Undetermined | NA | Death | |
| 34 + 1 | NA | No | Sepsis | Hemorrhagic meningoencephalitis, ventriculitis | Undetermined | NA | Death | |
| Heep et al., 2004 (68) (n = 1) | 27 | 950 | Intrauterine growth restriction, triplet pregnancy, intraventricular hemorrhage | Sepsis | Ventriculitis, hemorrhagic necrotizing lesions end brain abscesses | Central catheter | VCM, GTM, MRP | Recovery |
| Hilliard et al., 2003 (69) (n = 1) | 24 | 585 | Chorioamnionitis | Septic shock | No | Undetermined | VCM, TBM, CLD, MRP | Recovery |
| Chu et al., 2001 (70) (n = 1) | 26 | 1,580 | Hyaline membrane disease, CBD, patent ductus arteriosus, MV, parenteral nutrition | Sepsis | Meningitis, brain necrosis and edema | Undetermined | AMP, CFT, AMK, VCM | Death |
| Van Der Zwet et al., 2000 (71) (n = 3) | 28 + 5 | 895 | MV, patent ductus arteriosus | Septic shock | Hemorrhagic meningoencephalitis | Ventilator equipment, hands of medical staff | AMX, CFT | Death |
| 26+4 | 1,000 | MV | Sepsis | Knee arthritis | Ventilator equipment, hands of medical staff | VCM, MRP | Recovery | |
| 37+3 | 2,780 | MV, hepatosplenomegaly | Sepsis | Meningitis | Ventilator equipment, hands of medical staff | VCM, MRP | Recovery | |
| Tuladhar et al., 2000 (72) (n = 1) | 24 | 735 | MV, CNS hemorrhage | Sepsis | Bone marrow | Undetermined | CPF, CLD, GTM, IMP, VCM | Death |
| Tokieda et al., 1999 (73) (n = 1) | 37 | 3,764 | Hydrops fetalis, MV | Sepsis | Meningitis, brain hemorrhages | Peripheral catheter | AMP, GTM | Death |
| Jevon et al., 1993 (74) (n = 2) | 27 | 920 | Intrauterine growth restriction, hyaline membrane disease, MV, transfused transfusion syndrome, cerebral hemorrhage | Septic shock | Necrotizing pneumonia, necrosis of the larynx, thyroid and trachea, meningitis, subendocardial hemorrhage with focal necrosis | Resuscitation devices, drugs or hands of medical staff | AMP, GTM | Death |
| 25 | 690 | Hyaline membrane disease, MV, CNS hemorrhage | Septic shock | Necrotizing pneumonia | Resuscitation devices, drugs or hands of medical staff | AMP, GTM | Death | |
| Patrick et al., 1989 (75) (n = 1) | 26 | 830 | MV, thalamic hemorrhage | Septic shock | Meningitis, brain necrosis | Undetermined | AMK, VCM | Death |
| Turnbull et al., 1979 (77) (n = 1) | NA | NA | NA | Sepsis | Meningitis | Undetermined | NA | NA |
| Turnbull et al., 1977 (76) (n = 1) | 32 | 1,320 | Necrotizing enterocolitis | Sepsis | Brain, lung and respiratory tract necrosis | Central catheter | AMP, GTM | Death |
CBD, chronic bronchial disease; MV, mechanical ventilation; CNS, central nervous system; NA, not available.
VCM, vancomycin; AMK, amikacin; LNZ, linezolid; MRP, meropenem; AMP, ampicillin; TBM, tobramycin; PTZ, piperacillin/tazobactam; CTX, cefotaxime; CRP, chloramphenicol; FSF, fosfomycine; FCX, flucloxacilline; AMX, amoxicillin; GTM, gentamicin; CLD, clindamycin; AMP/SBT, ampicillin/sulbactam; PNP, panipenem; CFZ, cefazolin; MTZ, metronidazole; CPF, cefozopran; IMP, imipnem; CPF, ciprofloxacin; CFA, ceftazidime; ETY, erythromycin.
Other Severe Invasive Infections Caused by B. cereus in Neonates
Other types of severe invasive or localized infections without bacteremia in neonates have been recently reported in the literature. B. cereus can be responsible for severe neurological (78, 79), respiratory (83, 89), digestive (85, 90), and primary cutaneous infections (84). Recently, Viel-Thériault and colleagues have described a case of a neonate born at 26 weeks of gestational age who died of a rapidly progressive B. cereus necrotizing pneumonia following suspected nosocomial acquisition (89). Interestingly, B. cereus could also be associated with devastating intestinal infections, such as necrotizing enterocolitis (NEC). NEC is primarily a disease process of the GI tract of premature neonates that results in inflammation and bacterial invasion of the bowel wall. Despite advances in the care of premature infants, NEC remains one of the leading causes of morbidity and mortality in this population, with an incidence rate of 1% to 5% of all neonatal intensive care admissions (91). To date, the precise mechanisms involved in this disease are not fully understood. Several potential causes are often suggested, like diet, inflammation, or infection. An infectious etiology is suspected for NEC outbreaks. In their study, Wendelboe and colleagues described a cluster of NEC cases in a neonatal ICU in New Mexico in 2007 and suggested the potential involvement of B. cereus in this outbreak (90).
Finally, as previously suggested, it is important to note that all of these invasive infections related to B. cereus can occur in the form of epidemic nosocomial outbreaks in health care centers (2). Some of these outbreaks occur seasonally, with summer peaks, as shown in several studies (60, 92). These reports suggest a link between high ambient temperature, environmental dissemination, particularly within the hospital environment, and the epidemic spread of nosocomial infections. Indeed, these findings rely on the hypothesis that the growth of B. cereus is enhanced when temperatures are higher, which could explain the transient increase in number of infections especially during the summer.
Collectively, all these data suggest that premature infants are particularly susceptible to B. cereus invasive nosocomial infections. These infections can occur through bacterial transmission from the environmental reservoir of B. cereus to the neonate. The various environmental sources of infection in preterm neonates are discussed below.
ENVIRONMENTAL SOURCES OF INFECTION IN PRETERM NEONATES
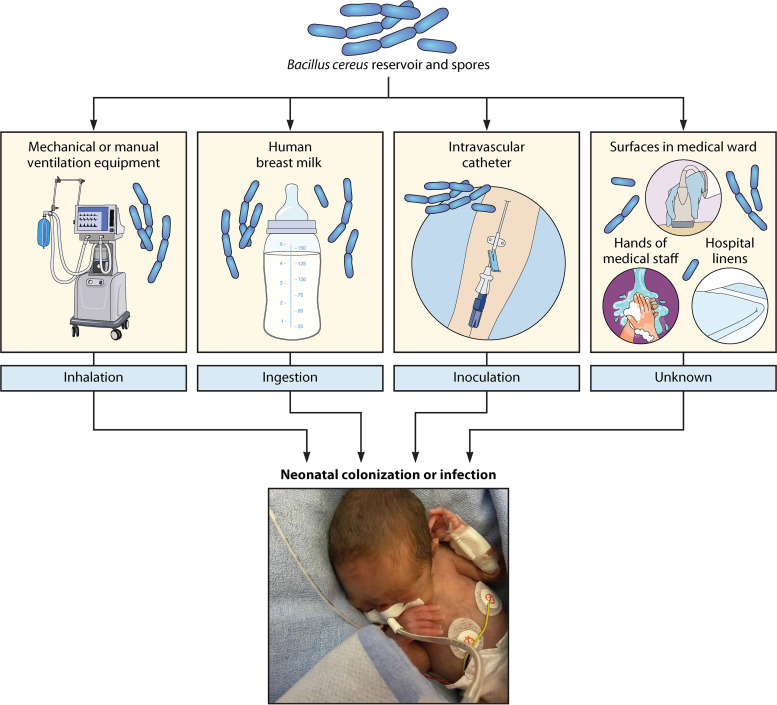
As previously suggested, B. cereus invasive infections can be fatal in preterm infants hospitalized in neonatal ICUs and sometimes even despite early and appropriate antimicrobial drug therapy (8, 51, 52, 70, 75). Considering the virulence potential of B. cereus in this population and the natural habitat of this bacterium in the environment, the question of the source of infection is of major concern in pediatric public health. Indeed, increasing our understanding of the origin of infection could help to prevent the transmission of B. cereus from the environmental reservoir and thus limit invasive infections in premature infants. In some studies, the authors were able to identify the source of contamination by showing a clonal link between patient and environmental strains. In preterm neonates, the main proven environmental sources of systemic infection are airborne contaminations through resuscitation devices (mechanical or manual ventilation equipment) (71, 83, 93) and inoculation via intravascular catheter (8, 53). Other environmental sources of infection have been described, including hospital linen, surfaces in medical wards, and hands of medical staff, and should be investigated in cases of B. cereus nosocomial outbreaks in pediatric ICUs (53, 58, 60, 71). The various suspected sources of infection that are investigated using various genotyping methods in cases of B. cereus invasive infections in neonates previously reviewed are summarized in Table 5 and Fig. 3.
TABLE 5.
Environmental sources of infections in neonates
| Suspected source of contaminationa (no. of cases) (references) | Proven source of contaminationb (%) (no. of proven cases/total no. of suspected cases) | Mode of transmission |
|---|---|---|
| Intravascular catheters (n = 5) (8, 53, 60, 68) | 60 (3/5) | Inoculation |
| Ventilator equipment (n = 17) (71, 83, 93) | 53 (9/17) | Inhalation |
| Human milk (n = 16) (7, 53, 54, 85) | 6 (1/16) | Ingestion |
| Surfaces in medical ward (n = 16) (8, 53, 58, 84) | 37 (6/16) | Unknown |
| Hands of medical staff (n = 5) (58, 71, 84) | 60 (3/5) | Unknown |
| Hospital linens (n = 3) (57, 60) | 33 (1/3) | Unknown |
| Parenteral nutritional solutions (n = 11) (7, 60); air, airborne dust, construction (n = 2) (58) | 0 (0/13) | Unknown |
Study of Bacillus cereus strains isolated from patients and hospital environments.
Same strain isolated from the patient and the hospital environment.
FIG 3.
Schematic representation of the various potential environmental sources of infection in preterm neonates infected by Bacillus cereus.
Enteral feeding contamination by B. cereus can result in either a GI infection or a bacteremia consecutive to gut translocation, especially in the context of neutropenia (94). Therefore, B. cereus invasive infection in neonatal ICUs associated with the ingestion of contaminated milk has been commonly suspected and investigated. In two different studies reporting severe intestinal infections related to B. cereus in preterm neonates, the putative role of contaminated, pulled breast milk has been suggested but not proven by typing of strains (85, 90). In another study, nine cases of B. cereus bacteremia were described in five neonatal resuscitation units (NRUs) of the APHP between August and December 2016 (7). Interestingly, B. cereus was isolated from various batches of pasteurized breast milk produced by the same manufacturers who delivered to the NRUs. However, a comparison of the strains showed great genotypic diversity and the implication of a batch contamination of the milk has not been proven. No common source could be identified. The lack of evidence regarding the origin of B. cereus in human breast milk was recently highlighted by a recent literature review (95). Recently, Liao and Tsai reported for the first time a case of B. cereus bacteremia in a preterm infant caused by consumption of contaminated breast milk using pulsed-field gel electrophoresis (54). Airborne contamination can occur by inhalation of B. cereus spores from contaminated air or dust or through the presence of contaminated respiratory equipment. Indeed, airflow sensors from contaminated mechanical ventilation devices have been proven to be responsible for outbreaks of B. cereus respiratory colonization (positive endotracheal aspiration) in newborns (71, 83, 93). Moreover, in other studies, the presence of construction work adjacent to the NRUs has led to dissemination of B. cereus spores in the air likely responsible for airborne contamination of premature neonates (51, 59, 66). Unfortunately, this last hypothesis has not been proven via molecular typing. Other potential sources of nosocomial infections caused by B. cereus, for example, contamination of gloves, hydroalcoholic gels, and hygiene procedure negligence in health care practices which could be responsible for the colonization of catheters, should not be neglected by the hygiene control team in cases of a nosocomial outbreak.
Finally, in most of the health care-associated infection clusters caused by B. cereus, a comparative analysis between clinical and environmental strains using various genotyping methods failed to prove the precise origin of the infection, as these strains were genotypically different. Indeed, the molecular investigations revealed that the clustered cases of infection were linked to a wide variety of bacterial clones (7, 8, 51, 94). The retrospective nature of the environmental sampling performed by hygiene control teams in cases of nosocomial outbreaks and the wide genetic diversity of B. cereus strains present in the environment could also explain the failure to identify the precise source of infection. We can therefore hypothesize that B. cereus can, from various elements of the environment, colonize the skin and the respiratory or digestive tract and under certain conditions (immunosuppression and/or acquisition of virulence factors) lead to true infection.
INVASIVE INFECTIONS IN NEONATES: PATHOPHYSIOLOGY
Given the emergence and potential severity of invasive infections caused by B. cereus in preterm neonates, it is crucial to understand the host-pathogen interactions and to decipher the molecular mechanisms involved in this disease. In this at-risk population, the severity of the infection can be explained by the high virulence potential of some strains of B. cereus or by a defect in the innate immune response capacity of the host, as premature neonates have an immature immune system, or by a combination of these two factors.
Virulence Factors of B. cereus sensu stricto
Some B. cereus sensu stricto strains produce several compounds that could contribute to their virulence associated with GI infections. The emetic toxin cereulide, present in food products, can be responsible for food intoxication characterized by emesis in humans. The pore-forming toxins hemolysin BL (HBL), nonhemolytic enterotoxin (NHE), and CytK can cause GI infections characterized by diarrhea (96, 97). The differential level of expression of the various potential enterotoxin genes could be a contributing factor to the broad spectrum of strain virulence. However, little is known about the virulence factors associated with non-GI invasive infections (32, 98, 99).
Recently, using a mouse model of infection, our team has shown that the pore-forming toxin α-hemolysin of Escherichia coli counteracts the innate immune response during bacteremia (100). Interestingly, the pore-forming HlyII of B. cereus has been shown to counteract the host immune system (101, 102) and is therefore suggested to play a role in invasive opportunistic infection. More recently, Mathur and colleagues (103) attempted to decipher the role of the multicomponent enterotoxin Hbl, which is highly conserved among B. cereus sensu stricto strains. The secretion of this toxin engages the activation of the NLRP3 inflammasome. Moreover, Hbl-producing B. cereus induces pyroptosis and cellular lysis in bone marrow-derived macrophages and rapid inflammasome-mediated mortality in a mouse model (C57BL/6). Furthermore, the authors showed that pharmacological inhibition of the NLRP3 inflammasome using MCC950 reduced the mortality of the mice infected by Hbl-producing B. cereus strains (103).
Together, these recent observations suggest that hypervirulent strains of B. cereus sensu stricto producing virulence factors such as HlyII or Hbl could be responsible for severe invasive infections in at-risk populations such as neonates. These studies also shed light on the potential relevance of pharmacological inhibitors of the inflammasomes as new drugs for the treatment of life-threatening bacterial infections.
Innate Immunity in Preterm Neonates
At birth, anti-infectious immunity is based mainly on the efficiency of the innate immune system (IS). Premature infants have immature ISs with reduced adaptive immune response capacities, which increases the risk of invasive infections. The preterm IS is also characterized by its lower capacity for neutralization and phagocytosis of infectious agents caused by a reduced activation of complement pathways, an impaired migration capacity of neutrophils to the site of infection, an impaired production of neutrophil extracellular traps (NETs), and also defective antigen presentation by monocytes and macrophages (104).
Premature birth is an increasing health care problem worldwide (105, 106). This increase in preterm births may partly account for the emergence of invasive bacterial infections in premature infants.
ANTIMICROBIAL SUSCEPTIBILITY TESTING AND ANTIBIOTIC TREATMENT
B. cereus (B. anthracis excluded) is frequently resistant to β-lactams, with the exception of carbapenems (47, 48, 51–53, 57, 66, 78, 87, 92, 94, 107–123). It is resistant to penicillin G and aminopenicillin, including ampicillin, ampicillin-sulbactam, amoxicillin, and amoxicillin-clavulanic acid. B. cereus is susceptible to third- and fourth-generation cephalosporins in 8% of cases (51 of 632 isolates) (53, 57, 66, 78, 87, 108, 109, 111, 112, 117, 119, 121, 122, 124). Little is known about the molecular mechanisms of antibiotic resistance of these bacteria. However, some authors have tried to decipher the main resistance mechanisms to β-lactams. B. cereus strains are intrinsically resistant to penicillins and cephalosporins by producing up to three chromosomal β-lactamases, named I, II, and III (125, 126). B. cereus β-lactamases I and III are serine-β-lactamases encoded by the blaI and blaIII genes, while β-lactamase II is a metallo-β-lactamase encoded by the blaII gene. Recently, Godič Torkar and Bedenic found that all 66 B. cereus isolates in their collection expressed blaII genes (122). Only two possessed the blaIII genes, and none possessed the blaI gene. If B. cereus strains are widely resistant to different classes of β-lactams including penicillins and cephalosporins, they are susceptible to various molecules that can be used as therapeutic agents as described below. Interestingly, carbapenems are active against B. cereus. Eighty-seven percent (254/290) and 94% (106/113) of the strains are susceptible to imipenem and meropenem, respectively. B. cereus is susceptible to glycopeptides, with 95% (575/606) and 100% (51/51) of the isolates being susceptible to vancomycin and teicoplanin, respectively (20, 47, 48, 51, 53, 57, 66, 69, 78, 87, 92, 94, 107–112, 114–119, 121–124, 127, 128). It is also susceptible to fluroquinolones, with 99% and 96% of the strains being susceptible to ciprofloxacin and levofloxacin, respectively (47, 48, 51, 53, 78, 87, 92, 94, 108–110, 112, 114, 115, 117–119, 121–123, 127). B. cereus is also susceptible to linezolid (57, 87, 92, 115–117, 123) and aminoglycosides (47, 48, 53, 57, 78, 87, 92, 107–109, 112, 115–121, 123, 124). In the literature, B. cereus has been reported to be frequently susceptible to macrolides and related antibiotics, with 75% (212/286), 85% (161/189), 89% (48/54), 74% (235/316), and 97% (29/30) of strains being susceptible to erythromycin, azithromycin, clarithromycin, clindamycin, and pristinamycin, respectively (47, 53, 57, 66, 78, 92, 107–112, 114, 117, 118, 121, 123, 124). All the current knowledge about in vitro antimicrobial susceptibility of B. cereus clinical isolates is reported in Table 6.
TABLE 6.
In vitro activity of antimicrobial agents against B. cereus clinical isolatesa
| Antibiotics | Susceptibility determined by AST guidelines (% susceptible strains) (no. of susceptible strains/total no.) |
Overall susceptibility |
Reference(s) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EUCAST (129) |
CLSI/NCCLS (130–135) |
|||||||||||||
| Staphylococcus spp. | Gram-positive bacteria | PK/PD breakpoints | Staphylococcus spp. | Bacillus spp. (not Bacillus anthracis) | Bacillus anthracis | Mycobacteria, nocardiae, other actinomycetes | Corynebacterium spp. | Gram-positive bacteria | Unspecified reference | Total no. of categorized strains | MIC range (mg/L) | % Susceptible | ||
| Beta-lactams | ||||||||||||||
| Penicillin G | 1 (2/137) | 0 (0/63) | 1 (1/67) | 0 (0/3) | 0 (0/126) | 396 | 0.012–>256 | 1 | 47, 48, 66, 78, 108–112, 116–119, 122–124 | |||||
| Ampicillin | 0 (0/8) | 2 (4/179) | 0 (0/1) | 0 (0/112) | 300 | 0.016–256 | 1 | 48, 53, 57, 87, 92, 107–112, 114, 115, 117, 123, 124 | ||||||
| Ampicillin - sulbactam | 4 (1/28) | 28 | <0.25–>8 | 4 | 87, 111 | |||||||||
| Amoxicillin | 4 (2/48) | 5 (2/42) | 90 | 0.016–>256 | 6 | 116, 117, 137 | ||||||||
| Amoxicillin – clavulanic acid | 22 (15/68) | 0 (0/90) | 158 | 0.5– 64 | 9 | 66, 112, 119, 124 | ||||||||
| Cloxacillin | 0 (0/60) | 60 | NA | 0 | 124 | |||||||||
| Oxacillin | 7 (3/43) | 43 | 0.094–>256 | 7 | 110, 117, 123 | |||||||||
| Ceftriaxone | 11 (9/82) | 5 (2/42) | 0 (0/1) | 125 | 8–>256 | 9 | 47, 66, 117, 122 | |||||||
| Cefotaxime | 1 (1/69) | 2 (2/78) | 28 (19/68) | 0 (0/112) | 327 | 0.1–>64 | 7 | 53, 78, 87, 108–112, 119, 122, 124 | ||||||
| Ceftazidime | 0 (0/69) | 0 (0/22) | 10 (2/20) | 111 | 2–>256 | 2 | 57, 87, 111, 121, 122 | |||||||
| Cefepime | 23 (16/69) | 69 | >32 | 23 | 94, 111, 122 | |||||||||
| Cefazolin | 0 (0/1) | 42 (34/80) | 81 | 1–64 | 42 | 48, 87, 92, 110 | ||||||||
| Imipenem | 0 (0/1) | 100 (2/2) | 100 (79/79) | 99 (135/136) | 100 (5/5) | 49 (33/67) | 290 | 0.004–>16 | 87 | 47, 48, 52, 53, 57, 66, 87, 92, 108, 110–115, 122 | ||||
| Meropenem | 94 (104/111) | 100 (1/1) | 100 (1/1) | 113 | 0.012–32 | 94 | 51, 57, 66, 111, 115, 117, 122 | |||||||
| Aminoglycosides | ||||||||||||||
| Amikacin | 100 (46/46) | 100 (1/1) | 100 (20/20) | 67 | 0.25–16 | 100 | 78, 87, 118, 121 | |||||||
| Gentamicin | 99 (124/125) | 99 (197/199) | 100 (1/1) | 100 (68/68) | 100 (101/101) | 494 | 0.016–16 | 99 | 47, 48, 53, 57, 78, 87, 92, 107–109, 112, 116–119, 122–124 | |||||
| Tobramycin | 100 (20/20) | 100 (1/1) | 100 (30/30) | 51 | 0.125–2 | 100 | 78, 112, 118 | |||||||
| Glycopeptides | ||||||||||||||
| Vancomycin | 100 (2/2) | 94 (136/144) | 98 (204/208) | 100 (1/1) | 87 (65/75) | 95 (167/176) | 606 | 0.06–24 | 95 | 20, 47, 48, 51, 53, 57, 69, 78, 87, 92, 94, 107–112, 114–122, 124, 127, 128 | ||||
| Teicoplanin | 100 (51/51) | 51 | 0.125–2 | 100 | 92, 110, 113 | |||||||||
| Cyclic lipopeptide | ||||||||||||||
| Daptomycin | 80 (68/85) | 16 (8/51) | 136 | 0.032–8 | 56 | 87, 92, 117, 118, 123, 127 | ||||||||
| Quinolones | ||||||||||||||
| Ciprofloxacin | 99 (75/76) | 98 (120/122) | 98 (131/133) | 100 (1/1) | 100 (1/1) | 100 (127/127) | 460 | 0.008–>4 | 99 | 47, 48, 53, 78, 94, 107–109, 111, 112, 114, 116–119, 121–123, 127 | ||||
| Levofloxacin | 100 (2/2) | 100 (20/20) | 92 (74/80) | 100 (60/60) | 162 | 0.064–32 | 96 | 47, 57, 87, 92, 108, 111, 115, 117, 123 | ||||||
| Macrolides and related | ||||||||||||||
| Azithromycin | 74 (49/66) | 100 (56/56) | 83 (56/67) | 189 | 0.016–128 | 85 | 53, 119, 122 | |||||||
| Clarithromycin | 98 (41/42) | 58 (7/12) | 54 | <0.12–4 | 89 | 108, 117 | ||||||||
| Clindamycin | 69 (9/13) | 80 (142/178) | 0 (0/1) | 100 (7/7) | 66 (77/117) | 316 | 0.032–16 | 74 | 47, 48, 53, 57, 66, 78, 87, 92, 107–111, 117, 121, 123 | |||||
| Erythromycin | 25 (1/4) | 72 (89/123) | 100 (1/1) | 78 (53/68) | 75 (68/90) | 286 | 0.032–24 | 75 | 48, 78, 87, 92, 107, 109, 110, 112, 114, 117–119, 124 | |||||
| Pristinamycin | 97 (29/30) | 30 | NA | 97 | 112 | |||||||||
| Cyclins | ||||||||||||||
| Doxycycline | 96 (46/48) | 48 | <0.03–16 | 96 | 116, 118 | |||||||||
| Tetracycline | 97 (70/72) | 100 (98/98) | 84 (56/67) | 100 (1/1) | 238 | 0.016–128 | 95 | 53, 107, 109, 111, 117, 119, 122, 123 | ||||||
| Tigecycline | 100 (1/1) | 100 (42/42) | 43 | 100 | 117, 123 | |||||||||
| Oxazolidinone | ||||||||||||||
| Linezolid | 100 (2/2) | 100 (116/116) | 94 (48/51) | 169 | 0.125–4 | 98 | 57, 87, 92, 115–117 | |||||||
| Others | ||||||||||||||
| Rifampicin | 94 (47/50) | 99 (113/114) | 100 (1/1) | 87 (26/30) | 195 | 0.002–8 | 96 | 53, 87, 107, 112, 114, 116, 117, 123 | ||||||
| Sulfamethoxazole-trimethoprim | 62 (8/13) | 72 (107/149) | 100 (1/1) | 163 | 0.064–>32 | 71 | 47, 48, 53, 92, 109, 114, 117, 123 | |||||||
AST, antimicrobial susceptibility testing; CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; NCCLS, National Committee for Clinical Laboratory Standards; PK/PD, pharmacokinetic/pharmacodynamic; NA, not available.
Of note, if guidelines for B. cereus spp. (exclusive of B. anthracis) were provided by the Clinical and Laboratory Standards Institute, only a few authors used these recommendations to perform antimicrobial susceptibility testing (AST) in their studies. All the AST results collected from the literature were obtained by using various AST methods, inocula, and clinical breakpoints (see Table 6 for details) (129–135).
Very recently, in April 2021, specific recommendations for Bacillus species (exclusive of B. anthracis) were established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for the standardization of AST methods and clinical breakpoint interpretation (136). AST can be performed using broth microdilution in Mueller-Hinton broth with an inoculum of 5 × 105 CFU/mL and incubation for 24 h under aerobic conditions or by the disk diffusion method with a MacFarland 0.5 inoculum. EUCAST also provides clinical breakpoints for AST interpretation for the following eight antibiotics: imipenem, meropenem, ciprofloxacin, levofloxacin, vancomycin, erythromycin, clindamycin, and linezolid.
As of today, no specific treatment guidelines concerning B. cereus-related infections have been published. In the literature and considering only patients for whom antibiotic therapy information was available, 63% (33/52) of neonates with B. cereus bacteremia were treated with antibiotic therapy, including intravenous vancomycin (8, 51–57, 59, 60, 62, 65, 66, 68–72, 75, 88). The treatment was vancomycin monotherapy in 12 cases out of 33 (36%), and all these patients had favorable outcomes. In the 21 cases of multitherapy including vancomycin, 12 patients with favorable outcomes received a wide variety of antibacterial combinations: bitherapy with amikacin (n = 1), meropenem (n = 4), ampicillin (n = 1), or cefotaxime (n = 1); tritherapy with aminoglycosides and β-lactams (cefotaxime, meropenem, or piperacillin-tazobactam) (n = 4); or a combination of four antibiotics for one patient (vancomycin, tobramycin, clindamycin, and meropenem). Among the 33% of patients treated with vancomycin, a total of 73% (24/33) had favorable clinical and microbiological outcomes (51–53, 55–58, 60, 62, 65, 66, 68–72, 75, 78, 79, 83, 85, 88). These findings, along with the large percentage of vancomycin-susceptible strains and the pharmacological properties of this antibiotic, make vancomycin the treatment of choice in sepsis caused by B. cereus. Furthermore, given the very high susceptibility rate of B. cereus strains to aminoglycosides, the synergistic action with glycopeptides, and the history of successful treatment of neonates with these two antibiotics classes, we could propose the association of vancomycin with an aminoglycoside as the treatment of choice for B. cereus invasive infections in neonates. β-Lactams are widely used in the treatment of sepsis or septic shock due to their wide antibacterial array and pharmacological properties but should not be recommended alone in the treatment of B. cereus invasive infections because of high MICs and history of treatment failures. In particular, 40% of neonates treated with β-lactams without vancomycin died in the days or weeks following the beginning of infection (60, 61, 64, 71, 73, 74, 76, 82, 89).
CONCLUSIONS
To date, the B. cereus group proposed in our study encompasses 16 validly published species that are involved in sepsis and other life-threatening infections in preterm neonates. Around 33% of these infections are fatal despite an early and appropriate wide-spectrum antibiotic treatment active against the isolated strains. The high virulence potential of some B. cereus strains and the host innate immune response play a crucial role in patient outcomes in this at-risk population. In preterm neonates, the main proven environmental sources of B. cereus and gateways to systemic infection are airborne contaminations through resuscitation devices (mechanical or manual ventilation equipment) (71, 83, 93) and inoculation via intravascular catheter (8, 53). A very recent study has shown for the first time a case of B. cereus bacteremia in a preterm infant caused by consumption of contaminated breast milk (54). Hygiene control teams should be particularly reactive in order to identify the previously known or new environmental sources of infants’ nosocomial infections and to avoid the epidemic spread of B. cereus clones in neonatal intensive care units. Of note, infections caused by B. cereus might have been overlooked since B. cereus isolated in human clinical samples has, for a long time, been regarded as a contaminant of the culture from the environmental reservoir. Our work questions the emergence or reemergence of these infections in preterm neonates. Furthermore, our review sheds light on recently described species thanks to advances in molecular identification methods using WGS. Interestingly, routine identification methods usually based on MALDI-TOF MS technology in routine clinical microbiology laboratories do not allow one to discriminate between these species. For example, the foodborne human pathogen B. cereus sensu stricto differs from B. thuringiensis, an insect pathogen widely used as a biopesticide, only by the presence or absence of the Cry toxin-encoding plasmid. In addition, B. paranthracis was recently involved in an emetic outbreak (30) and invasive infections in newborns (31). Therefore, B. paranthracis could have a high virulence potential, and further studies using NGS methods are required to understand whether the clinical strains implicated in the different B. cereus group infections belong to preferential and individual formerly known species or to newly described and emerging species.
Finally, in the absence of specific recommendations and given high MIC levels and treatment failure with β-lactams, this class of antibiotics should not be recommended alone for the treatment of B. cereus invasive infections. Given the information provided above (see Antimicrobial Susceptibility Testing and Antibiotic Treatment), we recommend the combination of vancomycin and aminoglycosides for the treatment of B. cereus group-related invasive infections in neonates before AST results.
ACKNOWLEDGMENTS
We are grateful to Abby Cuttriss, Université Côte d’Azur, for the careful reading and English editing of the manuscript. We warmly thank Florence Casagrande (Neonatal Intensive Care Unit, CHU) for permission to take the picture of a premature newborn hospitalized in the neonatal intensive care unit at the Nice teaching hospital in France shown in Fig. 3.
Biographies

Romain Lotte, Associate Professor (M.D., Ph.D.), Laboratory of Bacteriology, Nice University Hospital, Nice, France. Dr. Lotte is a medical doctor in the microbiology department at Centre Hospitalier Universitaire de Nice, Nice, France, and a clinical researcher at the National Institute for Health and Medical Research in France (Inserm). He is a member of the “Microbial Virulence and Inflammatory Signaling” team at the Mediterranean Center for Molecular Medicine (C3M, Inserm) at the University of Côte d'Azur, specializing in the innate immune response in the context of infectious diseases. During his Ph.D. training, he was trained in host responses to bacterial virulence factors. His primary research interests include infections by emerging pathogens, virulence of pathogens, and host innate immunity. He focuses especially on the mechanisms involved in the host-pathogen interaction during invasive infections caused by Bacillus cereus in preterm neonates.

Alicia Chevalier, Resident (M.D., Ph.D. student), Laboratory of Bacteriology, Nice University Hospital, Nice, France. Dr. Chevalier is a medical doctor in the microbiology department at Centre Hospitalier Universitaire de Nice, Nice, France, which is headed by Prof. Raymond Ruimy. She is also a Ph.D. student at the National Institute for Health and Medical Research in France (Inserm). She is a member of the “Microbial Virulence and Inflammatory Signaling” team headed by Dr. Laurent Boyer at the Mediterranean Center for Molecular Medicine (C3M, Inserm) at the University of Côte d'Azur, specializing in the innate immune response in the context of infectious diseases. For the last two years of her Ph.D. training, Dr. Chevalier’s research has focused on the identification, antibiotic resistance, and virulence of Bacillus cereus strains involved in invasive infections in preterm neonates.

Laurent Boyer, Research Director Inserm, team leader at the Mediterranean Center for Molecular Medicine (C3M), Nice, France. Dr. Boyer is a researcher at the National Institute for Health and Medical Research in France (Inserm). He specializes in the innate immune response in the context of infectious diseases. During his academic training, he was trained in host responses to bacterial virulence factors, and during his postdoctoral training at Harvard Medical School in the United States, he focused his work on the mechanisms of detection of virulence factors by the immune system. Since 2018, he has headed the “Microbial Virulence and Inflammatory Signaling” team at the Mediterranean Center for Molecular Medicine at the University of Côte d'Azur. This research team of more than 20 people brings together scientists specializing in molecular medicine with medical biologists and clinicians. The research theme of his laboratory is the study of the virulence of pathogens and the immune mechanisms of virulence sensing by the innate immune system.

Raymond Ruimy, Head of Department, Laboratory of Bacteriology, Nice University Hospital, Nice, France. Professor Raymond Ruimy (M.D., Ph.D.) is a professor of clinical bacteriology at the medical school of the University of Côte d’Azur (UCA), Nice, France. Since 2012, he has headed the laboratory of bacteriology at Centre Hospitalier Universitaire (CHU) Nice. He is also a clinical researcher at the National Institute for Health and Medical Research in France (Inserm) on the “Microbial Virulence and Inflammatory Signaling” team at the Mediterranean Center for Molecular Medicine (C3M). During his academic training, he was trained in bacterial phylogeny and evolution (University of Paris 6). He has been an associate professor of clinical bacteriology at the CHU Bichat-Claude Bernard (University of Paris 7). He has worked in the field of bacterial resistance to antibiotics in the One Health context. Since 2017, he has focused his research on the mechanisms involved in the host-pathogen interaction during invasive infections by emerging pathogens such as Bacillus cereus.
Contributor Information
Romain Lotte, Email: lotte.r@chu-nice.fr.
Raymond Ruimy, Email: ruimy.r@chu-nice.fr.
REFERENCES
- 1.Drobniewski FA. 1993. Bacillus cereus and related species. Clin Microbiol Rev 6:324–338. 10.1128/CMR.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson CJ, Kracalik IT, Ross N, Alexander KA, Hugh-Jones ME, Fegan M, Elkin BT, Epp T, Shury TK, Zhang W, Bagirova M, Getz WM, Blackburn JK. 2019. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat Microbiol 4:1337–1343. 10.1038/s41564-019-0435-4. [DOI] [PubMed] [Google Scholar]
- 4.Rasko DA, Altherr MR, Han CS, Ravel J. 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol Rev 29:303–329. 10.1016/j.femsre.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 5.European Food Safety Authority. European Centre for Disease Prevention and Control. 2021. The European Union One Health 2019 Zoonoses Report. EFSA J 19:6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall KK, Lyman JA. 2006. Updated review of blood culture contamination. Clin Microbiol Rev 19:788–802. 10.1128/CMR.00062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fournier S, Faraut-Derouin V, Casseta A, Frange P, Doit C, Fortineau N, Frange P, Doit C, Fortineau N, Romain O, Patkai J, de Chillaz C, Rigourd V, Baud O, Le Sache N, Blanchard Hervé, Bonacorsi S, Doucet-Populaire F, Bille E, Barnier JP, Nassif X, Batéjat C, Gohar M, Chamoin A, Herbin S, Berger-Carbonne A, Poyart C, Jarlier V. 2018. Bactériémies à Bacillus cereus en réanimation néonatale à l'AP-HP en 2016. Bull Epidémiol Hebd (25-26):536–540.
- 8.Lotte R, Hérissé A-L, Berrouane Y, Lotte L, Casagrande F, Landraud L, Herbin S, Ramarao N, Boyer L, Ruimy R. 2017. Virulence analysis of Bacillus cereus isolated after death of preterm neonates, Nice, France, 2013. Emerg Infect Dis 23:845–848. 10.3201/eid2305.161788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn F. 1872. Beiträge zur Biologie der Pfanzen, 1875 ed.
- 10.Frankland G, Frankland P. 1887. Studies on some new microorganisms obtained from air. Philos Trans R Soc Lond B Biol Sci 178:257–287. [Google Scholar]
- 11.Flügge C. 1886. Die Mikroorganismen Teil 1, auflage 3. Verlag von F.C.W. Vogel, Leipzig, Germany. [Google Scholar]
- 12.Berliner E. 1915. Uber die Schlaffsucht der Mehlmottenraupe (Ephestia kuhniella Zell.) und ihren Erreger, Bacillus thuringiensis n. sp. Z Angew Entomol 2:29–56. [Google Scholar]
- 13.Jiménez G, Urdiain M, Cifuentes A, López-López A, Blanch AR, Tamames J, Kämpfer P, Kolstø A-B, Ramón D, Martínez JF, Codoñer FM, Rosselló-Móra R. 2013. Description of Bacillus toyonensis sp. nov., a novel species of the Bacillus cereus group, and pairwise genome comparisons of the species of the group by means of ANI calculations. Syst Appl Microbiol 36:383–391. 10.1016/j.syapm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura L. 1998. Bacillus pseudomycoides sp. nov. Int J Syst Bacteriol 48:1031–1035. 10.1099/00207713-48-3-1031. [DOI] [PubMed] [Google Scholar]
- 15.Guinebretière M-H, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser M-L, Lamberet G, Fagerlund A, Granum PE, Lereclus D, De Vos P, Nguyen-The C, Sorokin A. 2013. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int J Syst Evol Microbiol 63:31–40. 10.1099/ijs.0.030627-0. [DOI] [PubMed] [Google Scholar]
- 16.Jung M-Y, Paek WK, Park I-S, Han J-R, Sin Y, Paek J, Rhee M-S, Kim H, Song HS, Chang Y-H. 2010. Bacillus gaemokensis sp. nov., isolated from foreshore tidal flat sediment from the Yellow Sea. J Microbiol 48:867–871. 10.1007/s12275-010-0148-0. [DOI] [PubMed] [Google Scholar]
- 17.Jung MY, Kim J-S, Paek WK, Lim J, Lee H, Kim PI, Ma JY, Kim W, Chang Y-H. 2011. Bacillus manliponensis sp. nov., a new member of the Bacillus cereus group isolated from foreshore tidal flat sediment. J Microbiol 49:1027–1032. 10.1007/s12275-011-1049-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller RA, Beno SM, Kent DJ, Carroll LM, Martin NH, Boor KJ, Kovac J. 2016. Bacillus wiedmannii sp. nov., a psychrotolerant and cytotoxic Bacillus cereus group species isolated from dairy foods and dairy environments. Int J Syst Evol Microbiol 66:4744–4753. 10.1099/ijsem.0.001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, Liu G-H, Hu G-P, Cetin S, Lin N-Q, Tang J-Y, Tang W-Q, Lin Y-Z. 2014. Bacillus bingmayongensis sp. nov., isolated from the pit soil of Emperor Qin’s terra-cotta warriors in China. Antonie Van Leeuwenhoek 105:501–510. 10.1007/s10482-013-0102-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Du J, Lai Q, Zeng R, Ye D, Xu J, Shao Z. 2017. Proposal of nine novel species of the Bacillus cereus group. Int J Syst Evol Microbiol 67:2499–2508. 10.1099/ijsem.0.001821. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Wang L, Han M, Xue Q, Zhang G, Gao J, Sun X. 2020. Bacillus fungorum sp. nov., a bacterium isolated from spent mushroom substrate. Int J Syst Evol Microbiol 70:1457–1462. 10.1099/ijsem.0.003673. [DOI] [PubMed] [Google Scholar]
- 22.Méndez Acevedo M, Carroll LM, Mukherjee M, Mills E, Xiaoli L, Dudley EG, Kovac J. 2020. Novel effective Bacillus cereus group species “Bacillus clarus” is represented by antibiotic-producing strain ATCC 21929 isolated from soil. mSphere 5:e00882-20. 10.1128/mSphere.00882-20. [DOI] [PMC free article] [PubMed]
- 23.Lechner S, Mayr R, Francis KP, Pruss BM, Kaplan T, Wiessner-Gunkel E, Stewart G, Scherer S. 1998. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int J Syst Bacteriol 48:1373–1382. 10.1099/00207713-48-4-1373. [DOI] [PubMed] [Google Scholar]
- 24.Glasset B, Herbin S, Guillier L, Cadel-Six S, Vignaud M-L, Grout J, Pairaud S, Michel V, Hennekinne J-A, Ramarao N, Brisabois A. 2016. Bacillus cereus-induced food-borne outbreaks in France, 2007 to 2014: epidemiology and genetic characterisation. Euro Surveill 21:30413. 10.2807/1560-7917.ES.2016.21.48.30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehling-Schulz M, Vukov N, Schulz A, Shaheen R, Andersson M, Märtlbauer E, Scherer S. 2005. Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus. Appl Environ Microbiol 71:105–113. 10.1128/AEM.71.1.105-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koehler TM, Dai Z, Kaufman-Yarbray M. 1994. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J Bacteriol 176:586–595. 10.1128/jb.176.3.586-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll LM, Cheng RA, Wiedmann M, Kovac J. 2021. Keeping up with the Bacillus cereus group: taxonomy through the genomics era and beyond. Crit Rev Food Sci Nutr 1–26. 10.1080/10408398.2021.1916735. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll LM, Wiedmann M, Kovac J. 2020. Proposal of a taxonomic nomenclature for the Bacillus cereus group which reconciles genomic definitions of bacterial species with clinical and industrial phenotypes. mBio 11:e00034-20. 10.1128/mBio.00034-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll LM, Wiedmann M, Mukherjee M, Nicholas DC, Mingle LA, Dumas NB, Cole JA, Kovac J. 2019. Characterization of emetic and diarrheal Bacillus cereus strains from a 2016 foodborne outbreak using whole-genome sequencing: addressing the microbiological, epidemiological, and bioinformatic challenges. Front Microbiol 10:144. 10.3389/fmicb.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben Khedher M, Nindo F, Chevalier A, Bonacorsi S, Dubourg G, Fenollar F, Casagrande F, Lotte R, Boyer L, Gallet A, Rolain J-M, Croce O, Ruimy R. 2021. Complete circular genome sequences of three Bacillus cereus group strains isolated from positive blood cultures from preterm and immunocompromised infants hospitalized in France. Microbiol Resour Announc 10:e00597-21. 10.1128/MRA.00597-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606. 10.1111/j.1574-6976.2008.00112.x. [DOI] [PubMed] [Google Scholar]
- 33.Guinebretière M-H, Thompson FL, Sorokin A, Normand P, Dawyndt P, Ehling-Schulz M, Svensson B, Sanchis V, Nguyen-The C, Heyndrickx M, De Vos P. 2008. Ecological diversification in the Bacillus cereus group. Environ Microbiol 10:851–865. 10.1111/j.1462-2920.2007.01495.x. [DOI] [PubMed] [Google Scholar]
- 34.Messelhäußer U, Ehling-Schulz M. 2018. Bacillus cereus—a multifaceted opportunistic pathogen. Curr Clin Microbiol Rep 5:120–125. 10.1007/s40588-018-0095-9. [DOI] [Google Scholar]
- 35.Wijnands LM, Dufrenne JB, Zwietering MH, van Leusden FM. 2006. Spores from mesophilic Bacillus cereus strains germinate better and grow faster in simulated gastro-intestinal conditions than spores from psychrotrophic strains. Int J Food Microbiol 112:120–128. 10.1016/j.ijfoodmicro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Vilas-Boas G, Sanchis V, Lereclus D, Lemos MVF, Bourguet D. 2002. Genetic differentiation between sympatric populations of Bacillus cereus and Bacillus thuringiensis. Appl Environ Microbiol 68:1414–1424. 10.1128/AEM.68.3.1414-1424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chon J-W, Song K-Y, Kim H, Seo K-H. 2014. Comparison of 3 selective media for enumeration of Bacillus cereus in several food matrixes. J Food Sci 79:M2480–M2484. 10.1111/1750-3841.12594. [DOI] [PubMed] [Google Scholar]
- 38.Pauker VI, Thoma BR, Grass G, Bleichert P, Hanczaruk M, Zöller L, Zange S. 2018. Improved discrimination of Bacillus anthracis from closely related species in the Bacillus cereus sensu lato group based on matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 56:e01900-17. 10.1128/JCM.01900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha M, Jo H-J, Choi E-K, Kim Y, Kim J, Cho H-J. 2019. Reliable identification of Bacillus cereus group species using low mass biomarkers by MALDI-TOF MS. J Microbiol Biotechnol 29:887–896. 10.4014/jmb.1903.03033. [DOI] [PubMed] [Google Scholar]
- 40.Manzulli V, Rondinone V, Buchicchio A, Serrecchia L, Cipolletta D, Fasanella A, Parisi A, Difato L, Iatarola M, Aceti A, Poppa E, Tolve F, Pace L, Petruzzi F, Rovere ID, Raele DA, Del Sambro L, Giangrossi L, Galante D. 2021. Discrimination of Bacillus cereus group members by MALDI-TOF mass spectrometry. Microorganisms 9:1202. 10.3390/microorganisms9061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruimy R, Breittmayer V, Elbaze P, Lafay B, Boussemart O, Gauthier M, Christen R. 1994. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int J Syst Bacteriol 44:416–426. 10.1099/00207713-44-3-416. [DOI] [PubMed] [Google Scholar]
- 42.Jensen GB, Hansen BM, Eilenberg J, Mahillon J. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ Microbiol 5:631–640. 10.1046/j.1462-2920.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 43.Grosh AC. 1978. Prevalence of Bacillus cereus in the faeces of healthy adults. J Hyg 80:233–236. 10.1017/S0022172400053572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stadhouders J, Hup G, Hassing F. 1982. The conceptions index and indicator organisms discussed on the basis of the bacteriology of spray-dried milk powder. Neth Milk Dairy J 36:231–260. [Google Scholar]
- 45.Ryu J-H, Beuchat LR. 2005. Biofilm formation and sporulation by Bacillus cereus on a stainless steel surface and subsequent resistance of vegetative cells and spores to chlorine, chlorine dioxide, and a peroxyacetic acid-based sanitizer. J Food Prot 68:2614–2622. 10.4315/0362-028x-68.12.2614. [DOI] [PubMed] [Google Scholar]
- 46.Inoue D, Nagai Y, Mori M, Nagano S, Takiuchi Y, Arima H, Kimura T, Shimoji S, Togami K, Tabata S, Yanagita S, Matsushita A, Nagai K, Imai Y, Takegawa H, Takahashi T. 2010. Fulminant sepsis caused by Bacillus cereus in patients with hematologic malignancies: analysis of its prognosis and risk factors. Leuk Lymphoma 51:860–869. 10.3109/10428191003713976. [DOI] [PubMed] [Google Scholar]
- 47.Tusgul S, Prod'hom G, Senn L, Meuli R, Bochud P-Y, Giulieri SG. 2017. Bacillus cereus bacteraemia: comparison between haematologic and nonhaematologic patients. New Microbes New Infect 15:65–71. 10.1016/j.nmni.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernaiz C, Picardo A, Alos JI, Gomez-Garces JL. 2003. Nosocomial bacteremia and catheter infection by Bacillus cereus in an immunocompetent patient. Clin Microbiol Infect 9:973–975. 10.1046/j.1469-0691.2003.00682.x. [DOI] [PubMed] [Google Scholar]
- 49.Marston CK, Ibrahim H, Lee P, Churchwell G, Gumke M, Stanek D, Gee JE, Boyer AE, Gallegos-Candela M, Barr JR, Li H, Boulay D, Cronin L, Quinn CP, Hoffmaster AR. 2016. Anthrax toxin-expressing Bacillus cereus isolated from an anthrax-like eschar. PLoS One 11:e0156987. 10.1371/journal.pone.0156987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, Mittal P, Gabor Than N, Espinoza J, Hassan SS. 2007. Recurrent preterm birth. Semin Perinatol 31:142–158. 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bar-Meir M, Kashat L, Zeevi DA, Well YW, Assous MV. 2019. A cluster of Bacillus cereus infections in the neonatal intensive care unit: epidemiologic and whole-genome sequencing Analysis. Pediatr Infect Dis J 38:e301–e306. 10.1097/INF.0000000000002441. [DOI] [PubMed] [Google Scholar]
- 52.Papan C, Förster K, Herterich R, Schulze A, Schubert S, Flemmer AW. 2019. Identification and containment of a cluster of two Bacillus cereus infections in a neonatal intensive care unit. Can J Infect Dis Med Microbiol 2019:1506583. 10.1155/2019/1506583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glasset B, Herbin S, Granier SA, Cavalié L, Lafeuille E, Guérin C, Ruimy R, Casagrande-Magne F, Levast M, Chautemps N, Decousser J-W, Belotti L, Pelloux I, Robert J, Brisabois A, Ramarao N. 2018. Bacillus cereus, a serious cause of nosocomial infections: epidemiologic and genetic survey. PLoS One 13:e0194346. 10.1371/journal.pone.0194346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao S-L, Tsai M-H. 2021. Bacillus cereus bacteremia in a preterm infant caused by consumption of contaminated breastmilk. Pediatr Neonatol 62:337–338. 10.1016/j.pedneo.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 55.Samarasekara H, Janto C, Dasireddy V, Polkinghorne A, Branley J. 2020. Bacillus cereus bacteraemia complicated by a brain abscess in a pre-term neonate. Access Microbiol 2:acmi000080. 10.1099/acmi.0.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramarao N, Belotti L, Deboscker S, Ennahar-Vuillemin M, de Launay J, Lavigne T, Koebel C, Escande B, Guinebretière MH. 2014. Two unrelated episodes of Bacillus cereus bacteremia in a neonatal intensive care unit. Am J Infect Control 42:694–695. 10.1016/j.ajic.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 57.Horii T, Tamai K, Notake S, Yanagisawa H. 2012. Bacillus cereus bloodstream infection in a preterm neonate complicated by late meningitis. Case Rep Infect Dis 2012:358789. 10.1155/2012/358789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimono N, Hayashi J, Matsumoto H, Mikaye N, Uchida Y, Shimoda S, Furusyo N, Akashi K. 2012. Vigorous cleaning and adequate ventilation are necessary to control an outbreak in a neonatal intensive care unit. J Infect Chemother 18:303–307. 10.1007/s10096-010-1072-2. [DOI] [PubMed] [Google Scholar]
- 59.Campbell JR, Hulten K, Baker CJ. 2011. Cluster of Bacillus species bacteremia cases in neonates during a hospital construction project. Infect Control Hosp Epidemiol 32:1035–1038. 10.1086/661910. [DOI] [PubMed] [Google Scholar]
- 60.Sasahara T, Hayashi S, Morisawa Y, Sakihama T, Yoshimura A, Hirai Y. 2011. Bacillus cereus bacteremia outbreak due to contaminated hospital linens. Eur J Clin Microbiol Infect Dis 30:219–226. 10.1007/s10096-010-1072-2. [DOI] [PubMed] [Google Scholar]
- 61.Saito M, Takahashi N, Ueda S, Kuwabara Y, Komiyama M, Koike Y, Yada Y, Honma Y, Momoi MY. 2010. Cytokine profile in a premature infant with systemic Bacillus cereus infection. Pediatr Int 52:e34–e36. 10.1111/j.1442-200X.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 62.Drazin D, Lehman D, Danielpour M. 2010. Successful surgical drainage and aggressive medical therapy in a preterm neonate with Bacillus cereus meningitis. Pediatr Neurosurg 46:466–471. 10.1159/000325073. [DOI] [PubMed] [Google Scholar]
- 63.Pawlik D, Lisowska-Miszczyk I, Radziszewska R, Ochoda A, Drzewiecki A, Podsiadło L, Pawlik W, Heczko P, Lauterbach R. 2009. A case of sepsis in ILBW infant caused by Bacillus cereus. Med Wieku Rozwoj 13:40–44. (In Polish). [PubMed] [Google Scholar]
- 64.Evreux F, Delaporte B, Leret N, Buffet-Janvresse C, Morel A. 2007. Méningite néonatale à Bacillus cereus, à propos d’un cas. Arch Pediatr 14:365–368. 10.1016/j.arcped.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 65.John AB, Razak EASA, Razak EEMH, Al-Naqeeb N, Dhar R. 2007. Intractable Bacillus cereus bacteremia in a preterm neonate. J Trop Pediatr 53:131–132. 10.1093/tropej/fml069. [DOI] [PubMed] [Google Scholar]
- 66.Adler A, Gottesman G, Dolfin T, Arnon S, Regev R, Bauer S, Litmanovitz I. 2005. Bacillus species sepsis in the neonatal intensive care unit. J Infect 51:390–395. 10.1016/j.jinf.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Lequin MH, Vermeulen JR, van Elburg RM, Barkhof F, Kornelisse RF, Swarte R, Govaert PP. 2005. Bacillus cereus meningoencephalitis in preterm infants: neuroimaging characteristics. AJNR Am J Neuroradiol 26:2137–2143. [PMC free article] [PubMed] [Google Scholar]
- 68.Heep A, Schaller C, Rittmann N, Himbert U, Marklein G, Bartmann P. 2004. Multiple brain abscesses in an extremely preterm infant: treatment surveillance with interleukin-6 in the CSF. Eur J Pediatr 163:44–45. 10.1007/s00431-003-1333-5. [DOI] [PubMed] [Google Scholar]
- 69.Hilliard NJ, Schelonka RL, Waites KB. 2003. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol 41:3441–3444. 10.1128/JCM.41.7.3441-3444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu W, Que T, Lee W, Wong S. 2001. Meningoencephalitis caused by Bacillus cereus in a neonate. Hong Kong Med J 7:89–92. [PubMed] [Google Scholar]
- 71.Van Der Zwet WC, Parlevliet GA, Savelkoul PH, Stoof J, Kaiser AM, Van Furth AM, Vandenbroucke-Grauls CM. 2000. Outbreak of Bacillus cereus infections in a neonatal intensive care unit traced to balloons used in manual ventilation. J Clin Microbiol 38:4131–4136. 10.1128/JCM.38.11.4131-4136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuladhar R, Patole S, Koh T, Norton R, Whitehall J. 2000. Refractory Bacillus cereus infection in a neonate. Int J Clin Pract 54:345–347. [PubMed] [Google Scholar]
- 73.Tokieda K, Morikawa Y, Maeyama K, Mori K, Ikeda K. 1999. Clinical manifestations of Bacillus cereus meningitis in newborn infants. J Paediatr Child Health 35:582–584. 10.1046/j.1440-1754.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- 74.Jevon G, Dunne W, Hicks M, Langston C. 1993. Bacillus cereus pneumonia in premature neonates: a report of two cases. Pediatr Infect Dis J 12:251–253. 10.1097/00006454-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Patrick CC, Langston C, Baker CJ. 1989. Bacillus species infections in neonates. Rev Infect Dis 11:612–615. 10.1093/clinids/11.4.612. [DOI] [PubMed] [Google Scholar]
- 76.Turnbull PC, French TA, Dowsett EG. 1977. Severe systemic and pyogenic infections with Bacillus cereus. Br Med J 1:1628–1629. 10.1136/bmj.1.6077.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turnbull PC, Jorgensen K, Kramer JM, Gilbert RJ, Parry JM. 1979. Severe clinical conditions associated with Bacillus cereus and the apparent involvement of exotoxins. J Clin Pathol 32:289–293. 10.1136/jcp.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lebessi E, Dellagrammaticas HD, Antonaki G, Foustoukou M, Iacovidou N. 2009. Bacillus cereus meningitis in a term neonate. J Matern Fetal Neonatal Med 22:458–461. 10.1080/14767050802610336. [DOI] [PubMed] [Google Scholar]
- 79.Manickam N, Knorr A, Muldrew K. 2008. Neonatal meningoencephalitis caused by Bacillus cereus. Pediatr Infect Dis J 27:843–846. 10.1097/INF.0b013e31816feec4. [DOI] [PubMed] [Google Scholar]
- 80.Weisse M, Bass J, Jarrett R, Vincent J. 1991. Nonanthrax Bacillus infections of the central nervous system. Pediatr Infect Dis J 10:243–246. 10.1097/00006454-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Feder H, Garibaldi R, Nurse B, Kurker R. 1988. Bacillus species isolates from cerebrospinal fluid in patients without shunts. Pediatrics 82:909–913. 10.1542/peds.82.6.909. [DOI] [PubMed] [Google Scholar]
- 82.Hendrickx B, Azou M, Vandepitte J, Jaeken J, Eggermont E. 1981. Bacillus cereus meningo-encephalitis in a pre-term baby. Acta Paediatr Belg 34:107–112. [PubMed] [Google Scholar]
- 83.Gray J, George RH, Durbin GM, Ewer AK, Hocking MD, Morgan MEI. 1999. An outbreak of Bacillus cereus respiratory tract infections on a neonatal unit due to contaminated ventilator circuits. J Hosp Infect 41:19–22. 10.1016/S0195-6701(99)90032-4. [DOI] [PubMed] [Google Scholar]
- 84.Saikia L, Gogoi N, Das PP, Sarmah A, Punam K, Mahanta B, Bora S, Bora R. 2019. Bacillus cereus—attributable primary cutaneous anthrax-like infection in newborn infants. Emerg Infect Dis 25:1261–1270. 10.3201/eid2507.181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Decousser J-W, Ramarao N, Duport C, Dorval M, Bourgeois-Nicolaos N, Guinebretière M-H, Razafimahefa H, Doucet-Populaire F. 2013. Bacillus cereus and severe intestinal infections in preterm neonates: putative role of pooled breast milk. Am J Infect Control 41:918–921. 10.1016/j.ajic.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 86.Girisch M, Ries M, Zenker M, Carbon R, Rauch R, Hofbeck M. 2003. Intestinal perforations in a premature infant caused by Bacillus cereus. Infection 31:192–193. 10.1007/s15010-002-3037-6. [DOI] [PubMed] [Google Scholar]
- 87.Ikeda M, Yagihara Y, Tatsuno K, Okazaki M, Okugawa S, Moriya K. 2015. Clinical characteristics and antimicrobial susceptibility of Bacillus cereus blood stream infections. Ann Clin Microbiol Antimicrob 14:43. 10.1186/s12941-015-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewin A, Delage G, Bernier F, Germain M. 2019. Banked human milk and quantitative risk assessment of Bacillus cereus infection in premature infants: a simulation study. Can J Infect Dis Med Microbiol 2019:6348281. 10.1155/2019/6348281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viel-Thériault I, Saban J, Lewis A, Bariciak E, Grynspan D. 2019. A case of fulminant Bacillus cereus lung necrosis in a preterm neonate. Pediatr Dev Pathol 22:461–464. 10.1177/1093526619825895. [DOI] [PubMed] [Google Scholar]
- 90.Wendelboe AM, Smelser C, Lucero CA, McDonald LC. 2010. Cluster of necrotizing enterocolitis in a neonatal intensive care unit: New Mexico, 2007. Am J Infect Control 38:144–148. 10.1016/j.ajic.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thompson AM, Bizzarro MJ. 2008. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs 68:1227–1238. 10.2165/00003495-200868090-00004. [DOI] [PubMed] [Google Scholar]
- 92.Kato K, Matsumura Y, Yamamoto M, Nagao M, Ito Y, Takakura S, Ichiyama S. 2014. Seasonal trend and clinical presentation of Bacillus cereus bloodstream infection: association with summer and indwelling catheter. Eur J Clin Microbiol Infect Dis 33:1371–1379. 10.1007/s10096-014-2083-1. [DOI] [PubMed] [Google Scholar]
- 93.Turabelidze G, Gee JE, Hoffmaster AR, Manian F, Butler C, Byrd D, Schildknecht S, Hauser LC, Duncan M, Ferrett R, Evans D, Talley C. 2013. Contaminated ventilator air flow sensor linked to Bacillus cereus colonization of newborns. Emerg Infect Dis 19:781–783. 10.3201/eid1905.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rhee C, Klompas M, Tamburini FB, Fremin BJ, Chea N, Epstein L, Halpin AL, Guh A, Gallen R, Coulliette A, Gee J, Hsieh C, Desjardins CA, Pedamullu CS, DeAngelo DJ, Manzo VE, Folkerth RD, Milner DA, Pecora N, Osborne M, Chalifoux-Judge D, Bhatt AS, Yokoe DS. 2015. Epidemiologic investigation of a cluster of neuroinvasive Bacillus cereus infections in 5 patients with acute myelogenous leukemia. Open Forum Infect Dis 2:ofv096. 10.1093/ofid/ofv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cormontagne D, Rigourd V, Vidic J, Rizzotto F, Bille E, Ramarao N. 2021. Bacillus cereus induces severe infections in preterm neonates: implication at the hospital and human milk bank level. Toxins 13:123. 10.3390/toxins13020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dietrich R, Jessberger N, Ehling-Schulz M, Märtlbauer E, Granum PE. 2021. The food poisoning toxins of Bacillus cereus. Toxins 13:98. 10.3390/toxins13020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Enosi Tuipulotu D, Mathur A, Ngo C, Man SM. 2021. Bacillus cereus: epidemiology, virulence factors, and host-pathogen interactions. Trends Microbiol 29:458–471. 10.1016/j.tim.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Ramarao N, Sanchis V. 2013. The pore-forming haemolysins of Bacillus cereus: a review. Toxins (Basel) 5:1119–1139. 10.3390/toxins5061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guinebretiere M-H, Broussolle V, Nguyen-The C. 2002. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J Clin Microbiol 40:3053–3056. 10.1128/JCM.40.8.3053-3056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diabate M, Munro P, Garcia E, Jacquel A, Michel G, Obba S, Goncalves D, Luci C, Marchetti S, Demon D, Degos C, Bechah Y, Mege J-L, Lamkanfi M, Auberger P, Gorvel J-P, Stuart LM, Landraud L, Lemichez E, Boyer L. 2015. Escherichia coli α-hemolysin counteracts the anti-virulence innate immune response triggered by the rho GTPase activating toxin CNF1 during bacteremia. PLoS Pathog 11:e1004732. 10.1371/journal.ppat.1004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tran SL, Ramarao N. 2013. Bacillus cereus immune escape: a journey within macrophages. FEMS Microbiol Lett 347:1–6. 10.1111/1574-6968.12209. [DOI] [PubMed] [Google Scholar]
- 102.Tran S-L, Guillemet E, Ngo-Camus M, Clybouw C, Puhar A, Moris A, Gohar M, Lereclus D, Ramarao N. 2011. Haemolysin II is a Bacillus cereus virulence factor that induces apoptosis of macrophages. Cell Microbiol 13:92–108. 10.1111/j.1462-5822.2010.01522.x. [DOI] [PubMed] [Google Scholar]
- 103.Mathur A, Feng S, Hayward JA, Ngo C, Fox D, Atmosukarto II, Price JD, Schauer K, Märtlbauer E, Robertson AAB, Burgio G, Fox EM, Leppla SH, Kaakoush NO, Man SM. 2019. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat Microbiol 4:362–374. 10.1038/s41564-018-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melville JM, Moss TJM. 2013. The immune consequences of preterm birth. Front Neurosci 7:79. 10.3389/fnins.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, Gülmezoglu AM. 2019. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 7:e37–e46. 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tielsch JM. 2015. Global incidence of preterm birth, p 9–15. In Embleton ND, Katz J, Ziegler EE (ed), Nestlé Nutrition Institute workshop series. S. Karger AG, Basel, Switzerland. [DOI] [PubMed] [Google Scholar]
- 107.Wright WF. 2016. Central venous access device-related Bacillus cereus endocarditis: a case report and review of the literature. Clin Med Res 14:109–115. 10.3121/cmr.2016.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uchino Y, Iriyama N, Matsumoto K, Hirabayashi Y, Miura K, Kurita D, Kobayashi Y, Yagi M, Kodaira H, Hojo A, Kobayashi S, Hatta Y, Takeuchi J. 2012. A case series of Bacillus cereus septicemia in patients with hematological disease. Intern Med 51:2733–2738. 10.2169/internalmedicine.51.7258. [DOI] [PubMed] [Google Scholar]
- 109.Lee Y-L, Shih S-D, Weng Y-J, Chen C, Liu C-E. 2010. Fatal spontaneous bacterial peritonitis and necrotizing fasciitis with bacteraemia caused by Bacillus cereus in a patient with cirrhosis. J Med Microbiol 59:242–244. 10.1099/jmm.0.011056-0. [DOI] [PubMed] [Google Scholar]
- 110.Kiyomizu K, Yagi T, Yoshida H, Minami R, Tanimura A, Karasuno T, Hiraoka A. 2008. Fulminant septicemia of Bacillus cereus resistant to carbapenem in a patient with biphenotypic acute leukemia. J Infect Chemother 14:361–367. 10.1007/s10156-008-0627-y. [DOI] [PubMed] [Google Scholar]
- 111.Ozkocaman V, Ozcelik T, Ali R, Ozkalemkas F, Ozkan A, Ozakin C, Akalin H, Ursavas A, Coskun F, Ener B, Tunali A. 2006. Bacillus spp. among hospitalized patients with haematological malignancies: clinical features, epidemics and outcomes. J Hosp Infect 64:169–176. 10.1016/j.jhin.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 112.Dubouix A, Bonnet E, Alvarez M, Bensafi H, Archambaud M, Chaminade B, Chabanon G, Marty N. 2005. Bacillus cereus infections in Traumatology-Orthopaedics Department: retrospective investigation and improvement of healthcare practices. J Infect 50:22–30. 10.1016/j.jinf.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 113.Koch A, Arvand M. 2005. Recurrent bacteraemia by 2 different Bacillus cereus strains related to 2 distinct central venous catheters. Scand J Infect Dis 37:772–774. 10.1080/00365540510012116. [DOI] [PubMed] [Google Scholar]
- 114.Carretto E, Barbarini D, Poletti F, Marzani FC, Emmi V, Marone P. 2000. Bacillus cereus fatal bacteremia and apparent association with nosocomial transmission in an intensive care unit. Scand J Infect Dis 32:98–100. 10.1080/00365540050164335. [DOI] [PubMed] [Google Scholar]
- 115.Horii T, Notake S, Tamai K, Yanagisawa H. 2011. Bacillus cereus from blood cultures: virulence genes, antimicrobial susceptibility and risk factors for blood stream infection. FEMS Immunol Med Microbiol 63:202–209. 10.1111/j.1574-695X.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 116.Mérens A, Vaissaire J, Cavallo J-D, Le Doujet C, Gros C, Bigaillon C, Paucod J-C, Berger F, Valade E, Vidal D. 2008. Etest for antibiotic susceptibility testing of Bacillus anthracis, Bacillus cereus and Bacillus thuringiensis: evaluation of a French collection. Int J Antimicrob Agents 31:490–492. 10.1016/j.ijantimicag.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 117.Luna VA, King DS, Gulledge J, Cannons AC, Amuso PT, Cattani J. 2007. Susceptibility of Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides and Bacillus thuringiensis to 24 antimicrobials using Sensititre(R) automated microbroth dilution and Etest(R) agar gradient diffusion methods. J Antimicrob Chemother 60:555–567. 10.1093/jac/dkm213. [DOI] [PubMed] [Google Scholar]
- 118.Citron DM, Appleman MD. 2006. In vitro activities of daptomycin, ciprofloxacin, and other antimicrobial agents against the cells and spores of clinical isolates of Bacillus species. J Clin Microbiol 44:3814–3818. 10.1128/JCM.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Turnbull PCB, Sirianni NM, LeBron CI, Samaan MN, Sutton FN, Reyes AE, Peruski LF. 2004. MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J Clin Microbiol 42:3626–3634. 10.1128/JCM.42.8.3626-3634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Godič Torkar K, Bedenić B, Plečko V. 2016. Antimicrobial susceptibility and the in vitro postantibiotic effects of vancomycin and ciprofloxacin against Bacillus cereus isolates. J Chemother 28:151–158. 10.1179/1973947815Y.0000000069. [DOI] [PubMed] [Google Scholar]
- 121.Callegan MC, Cochran DC, Kane ST, Ramadan RT, Chodosh J, McLean C, Stroman DW. 2006. Virulence factor profiles and antimicrobial susceptibilities of ocular Bacillus isolates. Curr Eye Res 31:693–702. 10.1080/02713680600850963. [DOI] [PubMed] [Google Scholar]
- 122.Godič Torkar K, Bedenić B. 2018. Antimicrobial susceptibility and characterization of metallo-β-lactamases, extended-spectrum β-lactamases, and carbapenemases of Bacillus cereus isolates. Microb Pathog 118:140–145. 10.1016/j.micpath.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 123.Ikram S, Heikal A, Finke S, Hofgaard A, Rehman Y, Sabri AN, Økstad OA. 2019. Bacillus cereus biofilm formation on central venous catheters of hospitalised cardiac patients. Biofouling 35:204–216. 10.1080/08927014.2019.1586889. [DOI] [PubMed] [Google Scholar]
- 124.Godič Torkar K, Seme K. 2009. Antimicrobial susceptibility, beta-lactamase and enterotoxin production in Bacillus cereus isolates from clinical and food samples. Folia Microbiol (Praha) 54:233–238. 10.1007/s12223-009-0037-2. [DOI] [PubMed] [Google Scholar]
- 125.Lim HM, Pène JJ, Shaw RW. 1988. Cloning, nucleotide sequence, and expression of the Bacillus cereus 5/B/6 beta-lactamase II structural gene. J Bacteriol 170:2873–2878. 10.1128/jb.170.6.2873-2878.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Y, Succi J, Tenover FC, Koehler TM. 2003. Beta-lactamase genes of the penicillin-susceptible Bacillus anthracis Sterne strain. J Bacteriol 185:823–830. 10.1128/JB.185.3.823-830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vodopivec I, Rinehart EM, Griffin GK, Johncilla ME, Pecora N, Yokoe DS, Feske SK, Milner DA, Folkerth RD. 2015. A cluster of CNS infections due to B. cereus in the setting of acute myeloid leukemia: neuropathology in 5 patients. J Neuropathol Exp Neurol 74:1000–1011. 10.1097/NEN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 128.Kalpoe JS, Hogenbirk K, van Maarseveen NM, Gesink-Van der Veer BJ, Kraakman MEM, Maarleveld JJ, van der Reyden TJK, Dijkshoorn L, Bernards AT. 2008. Dissemination of Bacillus cereus in a paediatric intensive care unit traced to insufficient disinfection of reusable ventilator air-flow sensors. J Hosp Infect 68:341–347. 10.1016/j.jhin.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 129.Société Française de Microbiologie. 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.1. CASFM/EUCAST European Committee on Antimicrobial Susceptibility Testing, Société Française de Microbiologie, Basel, Switzerland. [Google Scholar]
- 130.CLSI. 2006. Methods for antimicrobial dilution and disk susceptibility testing of Infrequently isolated or fastidious bacteria; approved guideline, 2nd ed. CLSI document M45-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 131.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 132.CLSI. 2015. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 3rd ed. CLSI guideline M45. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 133.CLSI. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 134.CLSI. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 135.National Committee for Clinical Laboratory Standards. 1984. Performance standards for antimicrobial disk susceptibility tests: approved standards M2-A. NCCLS, Villanova, PA. [Google Scholar]
- 136.Société Française de Microbiologie. 2021. Breakpoint tables for interpretation of MICs and zone diameters, version 1.0. CASFM/EUCAST European Committee on Antimicrobial Susceptibility Testing, Société Française de Microbiologie, Basel, Switzerland. [Google Scholar]
- 137.Barraud O, Hidri N, Ly K, Pichon N, Manea P, Ploy M-C, Garnier F. 2012. Pacemaker-associated Bacillus cereus endocarditis. Diagn Microbiol Infect Dis 74:313–315. 10.1016/j.diagmicrobio.2012.08.002. [DOI] [PubMed] [Google Scholar]