ABSTRACT
After the COVID-19 pandemic, vaccines using inactivated viruses have attracted worldwide attention for the prevention of infectious diseases. Here, we report a patient who suffered from Systemic Lupus Erythematosus (SLE) for six years and developed ocular symptoms within 72 hours after being vaccinated for COVID-19. The patient presented bilateral conjunctival congestion, eyelid edema with pruritus, and suffered from severe headaches. Recovery occurred within 10 days after the onset of symptoms after treatment with anti-infection drugs. The early identification and extensive assessment of side effects help ensuring effective vaccine safety monitoring.
KEYWORDS: COVID-19 vaccine, ocular adverse reactions, systemic lupus erythematosus, case report
1. Introduction
Phase III clinical trials evaluating COVID-19 vaccine efficiency started in 2020.1 However, the adverse events (AEs) of COVID-19 vaccination have not yet been comprehensively reported. This makes it necessary to understand the clinical manifestations of serious AEs caused by COVID-19 vaccination.2 The short-time frame on which vaccines have been available naturally reduces the sample size necessary to reliably detect rare AEs and clarify the mechanisms behind adverse vaccine reactions. Here, we report a case of ocular adverse reactions in temporal association with COVID-19 vaccination in a patient with Systemic Lupus Erythematosus (SLE).
2. Patient presentation
We describe a 28-year-old female suffering from SLE for six years and under chronic treatment with methylprednisolone (Medrol) 10 mg/d, mycophenolate mofetil (Cycopin) 1 g/d, tacrolimus (Prograf) 1.5 mg/d, and perindopril (Acertil) 4 mg/d. The patient received an inactivated COVID-19 vaccine on April 6, 2021. The patient developed a foreign body sensation, redness, eyelid edema with pruritus and severe headaches starting on April 9 and lasting until April 12, 2021 (Figure 1). Before vaccination, the patient had a history of high myopia, but no records of hypertension, organic ophthalmopathy or severe infections.
Figure 1.
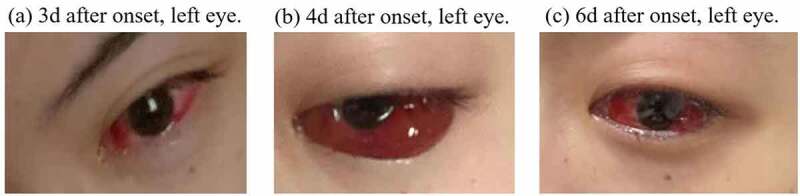
Images of left eye of a 28-year-old female who developed an acute ocular symptoms 3 days after COVID-19 vaccination.
(a) 3d after onset, left eye. (b) 4d after onset, left eye. (c) 6d after onset, left eye.
To ensure early diagnosis and treatment, the local hospital performed ophthalmic and blood examinations on the patient. The timeline sequence of symptoms and examinations is shown in Figure 2 and is described below.
Figure 2.
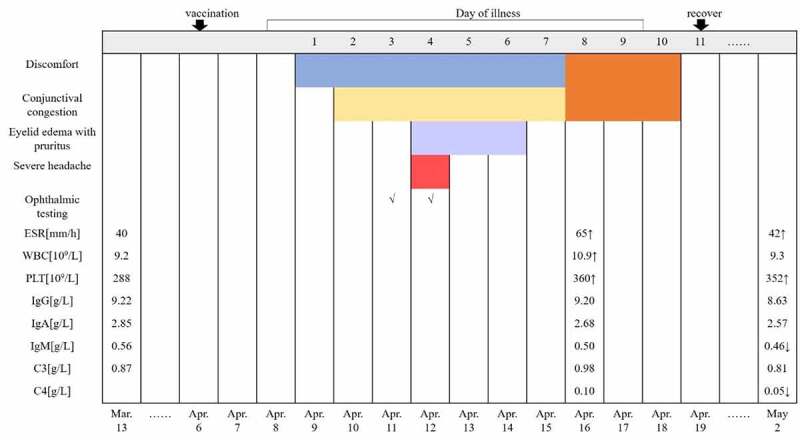
Disease timeline sequence of symptoms and examinations.
Abbreviations: ESR. Erythrocyte sedimentation rate; WBC. White blood cell; PLT. Platelet count; Ig. Immune globulin; C3. Complement 3; C4. Complement 4. ↑: Above normal; ↓: Below normal.
An eye examination performed on April 11 revealed: visual acuity, left 0.3 and right 0.2; intraocular pressure, left 17.5 mmHg and right 16.2 mmHg; bright corneas; normal depth of anterior chambers; and round pupils in both eyes. Funduscopy showed a standard fovea shape in both eyes. There was no evidence of organic eye disease. A day later (April 12), a second eye examination was performed and revealed the following findings: visual acuity, left 0.3 and right 0.2; intraocular pressure, left 16.6 mmHg and right 19.2 mmHg; corneal thickness, left 626 μm and right 575 μm; corneal endothelial cell density, left 2695/mm2 and right 2950/mm2; bright corneas; normal depth of anterior chambers; and round pupils in both eyes. Ultrasonographic images of the eyes revealed vitreous opacity and staphyloma of the posterior sclera in both eyes, but no obvious retinal detachment (Figure 3). A blood examination performed on April 16 showed the following: erythrocyte sedimentation rate (ESR) 65 mm/h, white blood cell (WBC) 10.9 × 109/L, platelet count (PLT) 360 × 109/L, serum amyloid A protein (SAA) 75.16 mg/L, IgG 9.20 g/L, IgA 2.68 g/L, IgM 0.50 g/L, C3 0.98 g/L, C4 0.10 g/L, C-reactive protein (CRP) 4.5 mg/L, serum ferriti (SF) 6.76 μmol/L, uric acid (UA) 383 μmol/L. A follow-up blood examination was performed on May 2 and showed: ESR 42 mm/h, WBC 9.3 × 109/L, PLT 352 × 109/L, SAA 15.44 mg/L, IgG 8.63 g/L, IgA 2.57 g/L, IgM 0.46 g/L, C3 0.81 g/L, C4 0.05 g/L, SF 5.13 μmol/L, UA 440 μmol/L.
Figure 3.
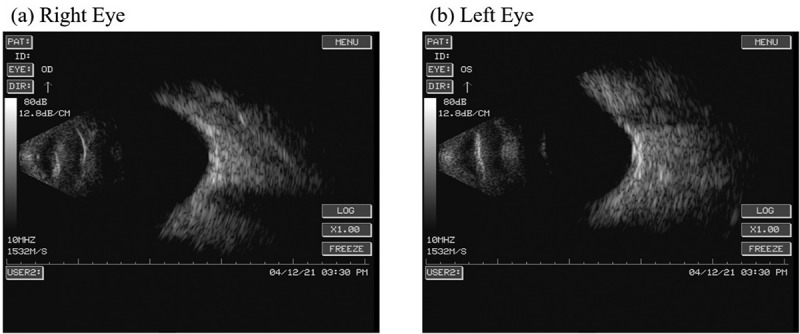
Ultrasonographic images of the eyes revealed vitreous opacity and staphyloma of the posterior sclera in both eyes, but no obvious retinal detachment.
(a) Right eye. (b) Left eye.
On the suspicion of acute hemorrhagic conjunctivitis, the patient was symptomatically treated with levofloxacin eye drops on the third day after symptom onset, when conjunctival congestion was already significant in the left eye. One day later, conjunctival congestion aggravated and was accompanied by the severe headaches and eyelid edema with pruritus. Therefore, diclofenac sodium eye drops and ganciclovir ophthalmic gel were added to the treatment. On the sixth day after symptom onset, conjunctival congestion and the swelling of the left eye gradually decreased but flumilone eye drops were added. On the eighth day after symptom onset, the patient gradually developed mild conjunctival congestion and pruritus in the right eye. Finally, the patient reported the lesions faded ten days after symptom onset.
3. Discussion
To the best of our knowledge, ocular adverse reactions associated with COVID-19 vaccine are rare. The ocular symptoms following vaccination reported in this study likely corresponds to an extremely rare event that coincided or might be associated with vaccination. We note that it is possible the patient has been exposed to an infectious agent or allergen before symptoms started, and that this caused the observed acute inflammatory response. However, the patient denied any history of allergies, recent infections and contact with anyone with COVID-19 infection, as well as similar ocular symptoms in her personal and family histories. The possibility of conjunctival congestion being induced by immune thrombocytopenia disease can be ruled out by the laboratory indicators.
Ocular hyperemia is a common complication in cases of acute exacerbation of SLE.3 However, the patient showed a stable SLEDAI score4 based on symptoms and laboratory indicators. This does not support the possibility of a vaccine-induced SLE disease flare. At present, however, it is not possible to rule out the infection was caused by vaccine organisms acting in an immunodeficient host.5 This could have stimulated the immune system to become hyperactive and increased the amount of certain autoantibodies or specific factors secreted by cells in the serum, which promoted the development of eye injury.6 This possibility and associated mechanisms merit further investigation.
The incidence of ocular inflammation after general vaccinations ranges between 8 to 13 in 100 000 people every year. The onset of symptoms usually occurs between 24 hours and 4 to 6 weeks after the vaccination.7 Most patients report feeling a foreign body in the eyes, conjunctival congestion or vision loss. The time of onset in this patient and the characteristics of the symptoms are consistent with the common reports from vaccine-induced ocular reactions. Since January 2021, Vaccine Adverse Event Reporting System (VAERS) has allowed publicly reported ocular AEs to be linked to COVID-19 vaccines.8 In a single month, there have been 46 cases of ocular- and adnexa-related side effects, of which a majority (34 [74%]) involved the eyelid or conjunctiva. These data indicate that we should raise enough awareness for possible ocular adverse reactions associated with COVID-19 vaccination.
4. Conclusions
Definite laboratory evidence establishing an association between vaccination and clinical manifestations is not yet available. However, the time interval between vaccination and the ocular adverse reactions reported here suggests a cause-and-effect relationship. This case highlights the need for closely monitoring the occurrence of conjunctival symptoms in patients with SLE after COVID-19 vaccination. Even SLE patients in stable stages of the disease should be monitored, especially when the immune index fluctuates. By enhancing awareness and recognition of possible adverse effects affecting the eye following vaccination, we can improve the safety and effectiveness of vaccination for patients with autoimmune diseases and minimize potential damage. The ocular symptoms presented here were reversible and eye function was not affected. Most ocular inflammation events occurring after COVID-19 vaccination thus subside without permanent visual impairment.
Funding Statement
No funding supported this study.
Highlights
Ocular lesions in patients with Systemic Lupus Erythematosus need to be monitored following COVID-19 vaccination.
Bilateral conjunctival congestion, eyelid edema with pruritus, and severe headaches may be associated with COVID-19 vaccination.
Ocular lesions were temporary and a combination of anti-infection and anti-inflammatory treatments helped recovery.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethical approval
The patient consent to the publication of this case report and accompanying image.
Author contributions
WJ: evidence collection, manuscript preparation and write the central part of the manuscript and manuscript revision.
YT: evidence collection, manuscript editing and revising.
CW: ideas, reviewed the manuscript.
All authors approved the final version of the manuscript.
References
- 1.Palacios R, Patiño EG, de Oliveira Piorelli R, Conde M, Batista AP, Zeng G, Xin Q, Kallas EG, Flores J, Ockenhouse CF, et al. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by sinovac - PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021;20:891–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silpa-Archa S, Lee JJ, Foster CS.. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 2016;100:135–41. doi: 10.1136/bjophthalmol-2015-306629. [DOI] [PubMed] [Google Scholar]
- 4.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rhumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 5.Bonilla FA. Update: vaccines in primary immunodeficiency. J Allergy Clin Immunol. 2018;141:474–81. doi: 10.1016/j.jaci.2017.12.980. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Zhang J, Zhou P, Sun H, Katsarou M, Drakoulis N. Exploration of vascular adhesion protein-1 expression in patients with conjunctivitis associated systemic lupus erythematosus using 2D-DIGE. Exp Ther Med. 2019;18:5072–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuniyoshi K, Hatsukawa Y, Kimura S, Fujino T, Ohguro H, Nakai R, Sunami K, Mishima SI, Sato T, Kusaka S, et al. Acute bilateral photoreceptor degeneration in an infant after vaccination against measles and rubella. JAMA Ophthalmol. 2017;135:478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng JY, Margo CE. Ocular adverse events following vaccination: overview and update. Surv Ophthalmol. 2021. doi: 10.1016/j.survophthal.2021.04.001. [DOI] [PubMed] [Google Scholar]


