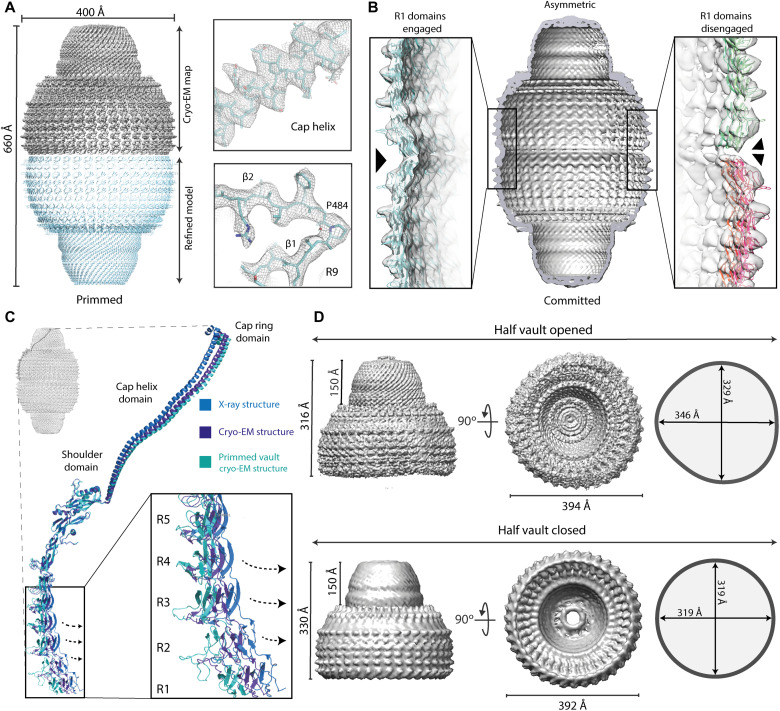
Fig. 2. Cryo-EM structures of multiple vault conformations in solution.
(A) Top left: Cryo-EM density for the high-resolution vault reconstruction with D39 symmetry imposed. Bottom: The corresponding refined atomic model derived from this map. Clear side chains could be identified in the map, especially around the cap helix and the R9 domain (right). (B) A second class of whole vault particles showed an asymmetrical configuration, with R1 domains from top and bottom half vaults engaged on one side (left) and disengaged at the other side (right). (C) While the overall conformation of MVP monomers is maintained in both symmetrical and asymmetrical reconstructions, the orientation of domains R1 to R5 relative to the N-terminal helix is changed, indicating a built-in structural variability in the whole vault particle affecting the volume of the internal cavities. (D) Top: A third reconstruction of free half vaults was identified in the datasets, exhibiting class distinctive architectural features, with a noncircular, oval contour. Dimensions for the cap and the waist cavity for the half vault reconstruction are indicated in two orthogonal views. For comparison, similar views from a map derived from the x-ray structure of the whole vault are shown at the bottom (PDB ID: 4HL8).