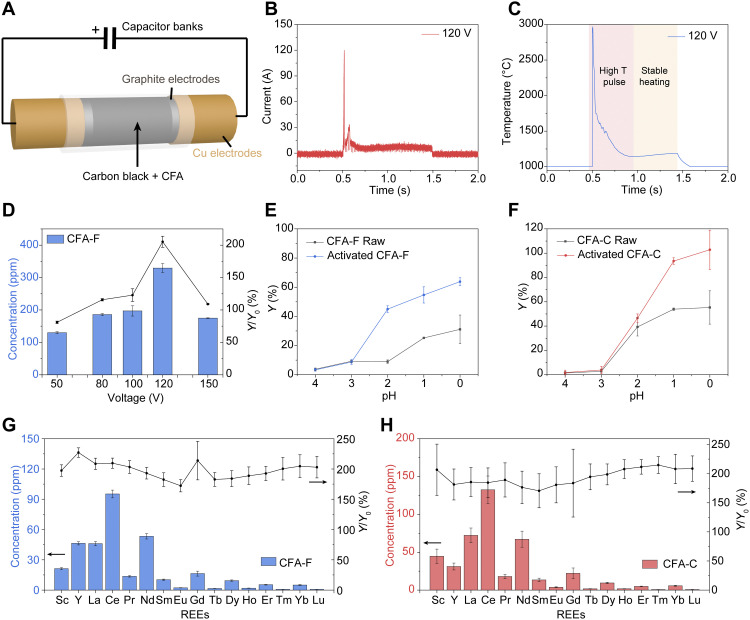
Fig. 2. Improved recovery yield of REE from CFA by electrothermal activation.
(A) Scheme of the FJH of CFA. (B) Current curve with the FJH condition of 120 V and 1 s. (C) Real-time temperature measurement with the FJH condition of 120 V and 1 s. (D) Relationship between HCl-leachable REE contents (1 M, 85°C) from activated CFA-F, increase in recovery yield (Y/Y0), and the FJH voltages. (E) pH-dependent REE leachability from the CFA-F raw materials and activated CFA-F. (F) pH-dependent leachability of REE from the CFA-C raw materials and activated CFA-C. (G) HCl-leachable individual REE contents (1 M, 85°C) from activated CFA-C and the increase in recovery yield. (H) HCl-leachable individual REE contents (1 M, 85°C) from activated CFA-F and the increase in recovery yield. Y0 represents the REE recovery yield by HCl leaching the CFA raw materials, and Y represents the REE recovery yield by HCl leaching the activated CFA. All error bars in (D) to (H) represent the SD, where N = 3.