Abstract
Background
Vaccination is an efficient strategy to control the COVID-19 pandemic. In north Cyprus, vaccine distribution started with CoronaVac followed by BNT162b2, and ChAdOx1 vaccines. An option to obtain a third booster dose with BNT162b2 or CoronaVac was later offered to people fully inoculated with CoronaVac. There are few simultaneous and comparative real-world antibody data for these three vaccines as well as boosters after CoronaVac vaccination. Our study was aimed at evaluating antibody responses after these vaccination schemes.
Methods
We did a prospective, longitudinal population-based study to measure SARS-CoV-2 anti-spike receptor binding domain (RBD) IgG concentrations, assessed by assaying blood samples collected, in participants in north Cyprus who had received the BNT162b2, ChAdOx1, or CoronaVac vaccine at 1 month and 3 months after the second dose. Participants were recruited when they voluntarily came to the laboratory for testing after vaccination, solicited from health-care access points, or from the general population. We also evaluated antibody responses 1 month after a booster dose of BNT162b2 or CoronaVac after primary CoronaVac regimen. Demographics, baseline characteristics, vaccination reactions, and percentage of antibody responders were collected by phone interviews or directly from the laboratory summarised by vaccine and age group. Antibody levels were compared between groups over time by parametric and non-parametric methods.
Findings
Recruitment, follow-up, and data collection was done between March 1 and Sept 30, 2021. BNT162b2 induced the highest seropositivity and anti-spike RBD IgG antibody titres, followed by ChAdOx1, and then by CoronaVac. In addition, the rate of decline of antibodies was fastest with CoronaVac, followed by ChAdOx1, and then by BNT162b2. For the older age group, the rate of seropositivity at 3 months after the second dose was 100% for BNT162b2, 90% for ChAdOx1, and 60% for CoronaVac. In the multivariate repeated measures model, lower antibody titres were also significantly associated with male sex, older age, and time since vaccination. Boosting a two-dose CoronaVac regimen at 6 months with a single BNT162b2 dose led to significantly increased titres of IgG compared with boosting with CoronaVac; for the 60 years and older age group, the geometric mean fold rise in antibody titre after the booster relative to 1 month post-baseline was 7·9 (95% CI 5·8–10·8) in the BNT162b2 boost group versus 2·8 (1·6–5·0) in the CoronaVac group.
Interpretation
These longitudinal data can help shape vaccination strategies. Given the low antibody titres and fast decline in the CoronaVac group in individuals 60 years or older, more potent vaccine options could be considered as the primary vaccination or booster dose in these high-risk populations to sustain antibody responses for longer.
Funding
Crowdfunded in north Cyprus.
Introduction
SARS-CoV-2, the virus behind the COVID-19 pandemic, has caused more than 5·5 million deaths by mid-January 2022. Various vaccines are currently available, aimed at controlling the COVID-19 pandemic. The most commonly used vaccine types are mRNA (BNT162b2 and mRNA-1273), adenoviral vector-based (ChAdOx1 nCoV-19, Ad26.CoV2.S, and Gam-COVID-Vac) or inactivated virus (CoronaVac and Sinopharm) vaccines. Several studies have highlighted the differing efficacy of these vaccines.1, 2, 3, 4, 5, 6, 7 The BNT162b2 (from Pfizer and BioNTech) vaccine has shown a 95% efficacy, and ChAdOx1 (from the University of Oxford and AstraZeneca) has shown a 70·4% efficacy in phase 3 clinical trials.4, 7 The European Medicines Agency has approved both these vaccines for use against COVID-19. For CoronaVac (from Sinovac), Turkey reported an 83·5% efficacy6 and Chile reported a 65·9% efficacy,2 and WHO issued approval for this vaccine, estimating its efficacy at 51%. Although these differences might be attributable to different populations or variants circulating in those regions at the time, more studies are needed to establish the efficacy and length of protection by this vaccine in real-world settings. WHO recommended that countries administering CoronaVac should implement their own studies evaluating the immunogenicity and efficacy of the vaccine in older age groups (>60 years).
Research in context.
Evidence before this study
The COVID-19 pandemic prompted scientific rigour in vaccine discovery, followed by a fast rollout of several SARS-CoV-2 vaccines including BNT126b2 from Pfizer and BioNtech, ChAdOx1 by AstraZeneca and The University of Oxford, and CoronaVac by Sinovac, among others. Many phase 2 and 3 studies have shown different rates of reactogenicity and efficacy for each vaccine. Although all vaccines led to varying rates of seropositivity in different cohorts, and conferred varying degrees of efficacy and protection, the timeline and extent of seropositivity in real life conditions are unclear. Additionally, WHO has recommended countries using CoronaVac do their own assessments in older age groups, because the data available were deemed insufficient. Furthermore, concerns on waning vaccine immunity brought along the prospect of vaccine boosts. BNT126b2 and ChAdOx1 third booster doses were shown to lead to efficient neutralisation. However, as of September, 2021, the third booster dose after primary vaccination with CoronaVac, and choice of vaccine boosts, have not been addressed. Considering the urgent global need for accessible vaccines and need for long-lasting, efficient protection in the face of SARS-CoV-2 variants, it is imperative to elucidate immunogenicity both after same-vaccine and different-vaccine boosters. NCBI was searched using key words such as “COVID-19”, “SARS-CoV-2”, “COVID-19 vaccines”, “phase 1/2/3 trials”, and “anti-Spike IgG” between July and September, 2021, where primarily research articles in English were considered.
Added value of this study
This study is the first prospective analysis comparing antibody titres longitudinally among three different SARS-CoV-2 vaccine types. Our study presents the real-life data of longitudinal SARS-CoV-2 anti-spike receptor binding domain (RBD) IgG titres upon vaccination with three different vaccine types, the mRNA-based BNT126b2, the adenoviral vector-based ChAdOx1, and the inactive virus vaccine CoronaVac. Our data present significant differences in IgG titres among vaccines, where a decrease in IgG titres was observed over time for all. However, the reduction in IgG titres and seroreversion was the most prominent for the CoronaVac vaccine, which also had the lowest IgG titres at the initial timepoint. Our data, for the first time, reveal real-life immunostimulatory effects associated with third booster vaccine doses; following a primary CoronaVac vaccination with either a third, single dose of CoronaVac or of BNT126b2. Immune stimulation, as established by SARS-CoV-2 anti-spike RBD IgG titres, was the highest when primary two-dose CoronaVac was boosted with BNT126b2 for the older age group.
Implications of all the available evidence
As vaccination programmes ramp up globally, there is an increased need for longitudinal data on vaccine immunogenicity, third doses, and mixing vaccines. Our study suggests that although IgG titres stimulated by all tested vaccines reduce over time, CoronaVac-induced IgG titres for older age groups (≥60 years) start lower and are markedly reduced over time, with 60% seropositivity observed at 3 months of follow-up. Here, we present compelling real-life evidence suggesting that the mixing and matching of vaccine doses, in particular a primary vaccination with full dose CoronaVac followed by a BNT126b2 booster, leads to high titres of SARS-CoV-2 anti-spike RBD IgG. Our results suggest that a booster dose with BNT126b2 in individuals fully vaccinated with CoronaVac could provide increased immune stimulation that could translate to a more robust immune response and protection.
During natural COVID-19 infection, antibodies act as the first line of defence where their levels can be affected by factors such as disease severity and comorbidities.8 Antibodies against the SARS-CoV-2 spike protein, and particularly that of the receptor binding domain (RBD), are thought to play an important role in priming the immune response as well as neutralising the virus.9
Particularly because the virus keeps spreading and evolving, various variants are expected to emerge with an ability to evade immune responses.10, 11 Studies have highlighted the importance of high spike antibody titres in mounting a protective immune response against SARS-CoV-2 infection.12 Studies also show that the occurrence of breakthrough infections correlates with spike RBD IgG antibody titres, further emphasising the direct relevance of antibody titres for protection against SARS-CoV-2 infection.13 Furthermore, it is unclear how long the immune response of the different vaccines will last, and whether additional dose(s) will need to be administered to boost the efficacy of available vaccines. As of September 2021 (time of writing), booster doses for all vaccines have been initiated worldwide where vaccines are available, 4–6 months after the second dose.
Although the first waves of the pandemic were managed well in north Cyprus,14 the new waves are causing higher case numbers and affecting the health-care system. Sequencing to establish variants of concern is not frequently done because of cost and inadequate infrastructure,14 making it difficult to attribute the cases to different variants. Although the efficacy of all vaccines appears to be reduced against the delta (B.1.617.2) and omicron (B.1.1.529) variants, BNT162b2, ChAdOx1, and others have shown high protection against COVID-19-related admissions to hospital and deaths.15
Vaccination in north Cyprus started with CoronaVac being offered to those older than 65 years and health-care workers, followed by BNT162b2 in those older than 65 years and ChAdOx1 in the 55–65 year group, with a subsequent expansion to younger age groups. Later, north Cyprus began administering a single booster dose of BNT162b2 to health-care workers and people older than 60 years who were originally fully vaccinated with CoronaVac. During this period, some individuals chose CoronaVac as their third dose, mostly because of a preconceived fear of novel technology as well as a fear of mixing vaccine technologies.
We investigated the longitudinal anti-spike RBD IgG antibody responses after vaccination with CoronaVac, BNT162b2, and ChAdOx1, as well as the effect of a third booster dose with BNT162b2 or CoronaVac after a full regimen of CoronaVac, with the aim of evaluating the titre and duration of antibody responses elicited by each vaccination scheme. To the best of our knowledge, this is the first study to prospectively and comparatively analyse the anti-spike RBD IgG antibody titres in an inactive (CoronaVac), an mRNA-based (BNT162b2), and an adenoviral vector-based (ChAdOx1) vaccine, in addition to evaluating a full dose of CoronaVac boosted at 6 months with BNT162b2 or CoronaVac, taking into consideration various baseline health variables including age, sex, body-mass index (BMI), and chronic diseases.
Methods
Study design and participants
We aimed to do a prospective, longitudinal population-based study to examine SARS-CoV-2 anti-spike RBD IgG concentrations after BNT162b2, ChAdOx1, and CoronaVac vaccines or after a booster dose, done in north Cyprus. At the time we planned the study, the vaccination plan for north Cyprus was not finalised and there was little information regarding which vaccines would be administered over time because this was heavily dependent on vaccine availability, regional and country allocations, and logistics. Because of anticipated varied immune responses in older (≥60 years) versus younger (<60 years) age populations, we planned to recruit participants (approximately 100, depending on availability) from each of these age groups per primary vaccine type who had received two doses of each vaccine for the main cohort. Primary assessments were targeted for 1 month and 3 month timepoints after the second dose of each vaccine. The time interval between the two doses of vaccine was 4 weeks for CoronaVac, 3 weeks for BNT162b2, and 12 weeks for ChAdOx1.
We also evaluated these cohorts: a subset of participants who initially received two doses of CoronaVac that were subsequently (approximately 6 months after the first dose) offered BNT162b2 or CoronaVac as a booster, and evaluated at 1 month post-booster; and an independent small reference group of participants who recovered from COVID-19 in the past 3 months and who had not received any vaccines.
The primary methods for recruitment were inviting the participants who voluntarily came to the laboratory for antibody testing after vaccination, and soliciting study participants from particular vaccine and age groups at health-care access points (public and private hospitals and laboratories) or from the general population via word of mouth. All participants provided informed consent acknowledging voluntary participation, an option to withdraw from study anytime, and the confidentiality of their antibody results and responses to the survey administered to obtain information on participant characteristics and reported vaccine reactions. The study has been approved by the Dr Burhan Nalbantoğlu State Hospital Ethics Committee in Nicosia, Cyprus.
Procedures
Blood collection was done between March and September, 2021, in two centres (Etik Hospital, Nicosia, Cyprus, and Dünya IVF Clinic, Kyrenia, Cyprus) and two private laboratories (Mikrolab, Nicosia, Cyprus, and Duolab, Kyrenia, Cyprus), and in a small proportion (n=14) it was implemented via home visits to a different region (Famagusta, Cyprus) to increase the regional diversity of the samples. Anti-SARS-CoV-2 spike RBD IgG was then measured. The samples were primarily analysed at Etik Hospital biochemistry laboratory, with a smaller subset of the samples (n=26) analysed at Microlab and Duolab that used the same method, kit, and device, and whose results were cross-verified against Etik Hospital's laboratory results using identical samples at study start. At study start, the assay VIDAS SARS-CoV-2 IgG (Biomerieux, Lyon, France) had European CE marking and US emergency use authorisation from the US Food and Drug Administration. Per the assay's recommended definition, the positive anti-spike RBD IgG response in the study was defined as a test value of 1·0 index or more, and the reported assay specificity was 99·9% (from the package insert). Assays were run on VIDAS Mini Vidas Blue System.
Telephone calls were made by the members of our team (authors and acknowledged members) to do a survey and obtain information on demographics, baseline characteristics, and vaccine reactogenicity between March and September, 2021. Telephone calls were made upon completion of both vaccine doses for the BNT162b2 and CoronaVac groups. Because of the greater spread between the two vaccine doses of ChAdOx1 in north Cyprus (12 week interval), survey details for this group were obtained after the first vaccine dose.
Statistical analysis
Primary outcomes were the percentage and magnitude of SARS-CoV-2 anti-spike RBD IgG antibody responses post-vaccination over time by vaccine and age group, and the secondary outcome was vaccine reactogenicity. Varying vaccine administration policy over time was controlled for by recruiting to and analysing the three main vaccine groups in both age groups of younger than 60 years and 60 years or older. Demographics, baseline characteristics, and vaccination reactions were summarised in a descriptive manner by vaccine and age groups. The percentage of antibody responders were summarised by vaccine, age group, and blood collection and testing timepoint. Antibody concentrations were evaluated using both parametric and non-parametric methods. Participants who reported to be on chemotherapy, corticosteroids, or immunosuppression (n=6) were removed from the antibody analyses, and participants who were diagnosed with COVID-19 by PCR testing during the study were censored after the time of diagnosis (n=3 with no 3 month testing after COVID-19 diagnosis). The overall comparison of antibody concentrations between vaccine groups was conducted by the Wilcoxon two-sample or Kruskal-Wallis test. Demographic and baseline predictors of antibody concentrations over the 3 month period after the second dose of vaccination were examined using univariate (only adjusting for vaccine group) and multivariate linear repeated measures models. For normality of the response variable, a log10 transformation was used. Within-person correlation of responses was considered via the use of an unstructured covariance matrix. All variables with p<0·1 from the univariate model were included in an initial multivariate model. Collected potential confounders (sex, age, BMI, health-care worker or not, blood type, blood Rhesus factor, chronic disease presence and type, allergies, pregnancy, smoking, physical health, and mental health) were adjusted for in the univariate and multivariate linear repeated measures models. The primary interaction of interest in these models was time-by-vaccine group interaction. The final model was established via the use of Akaike's Information Criterion. No missing data imputation was implemented for the small proportion of participants who could not come for their 3 month testing, since the reasons were non-health related and did not introduce bias. The robustness of results was verified via sensitivity analyses including the evaluation of different models and covariance structures, alternate parametrisation for the continuous factors of age and BMI, and the evaluation of consistency in trends across and within vaccine types, both visually and via non-parametric methods. Statistical significance was defined as p<0·05 except for pairwise comparisons among the three primary vaccine groups where Bonferroni adjustment was used (p<0·0167). Analyses were performed using SAS version 9.4, and figures were generated using GraphPad Prism version 9.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
In the primary vaccine groups in north Cyprus from March 1 to Sept 30, 2021, we evaluated a total of 384 participants (table 1 ), with 222 in the CoronaVac, 106 in the BNT162b2, and 56 in the ChAdOx1 groups. The median age in these groups was higher in BNT162b2 than in CoronaVac, and was lowest in ChAdOx1; the percentage of female participants was highest for CoronaVac, then ChAdOx1, then BNT162b2. Most health-care workers were in the CoronaVac group. The percentage of participants with a reported chronic disease was similar across the three groups. The most frequently reported chronic conditions were hypertension (35·7%), an autoimmune disease (23·4%), cardiovascular disease (21·4%), and diabetes (20·3%). A summary of detailed baseline characteristics by vaccine and age groups is included in table 1.
Table 1.
Demographics and baseline characteristics by vaccine and age groups
|
BNT162b2 |
ChAdOx1 |
CoronaVac |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age <60 years (n=24) | Age ≥60 years (n=82) | All (n=106) | Age <60 years (n=31) | Age ≥60 years (n=25) | All (n=56) | Age <60 years (n=95) | Age ≥60 years (n=127) | All (n=222) | ||
| Female | 11 (45·8%) | 43 (52·4%) | 54 (50·9%) | 19 (61·3%) | 13 (52·0%) | 32 (57·1%) | 65 (68·4%) | 79 (62·2%) | 144 (64·9%) | |
| Male | 13 (54·2%) | 39 (47·6%) | 52 (49·1%) | 12 (38·7%) | 12 (48·0%) | 24 (42·9%) | 30 (31·6%) | 48 (37·8%) | 78 (35·1%) | |
| Age, years | 42·5 (39·0–48·5) | 70·0 (66·0–74·0) | 68·0 (62·0–73·0) | 56·0 (50·0–57·0) | 62·0 (61·0–63·0) | 59·0 (55·0–62·0) | 38·0 (30·0–46·0) | 70·0 (65·0–75·0) | 63·5 (41·0–71·0) | |
| Body-mass index | 24·5 (21·2–28·6) | 27·3 (25·3–31·0) | 26·8 (24·2–30·4) | 27·3 (23·2–31·2) | 26·7 (25·4–28·5) | 27·0 (24·5–29·4) | 24·8 (22·2–27·5) | 27·7 (24·7–30·4) | 26·4 (23·7–29·4) | |
| Health-care worker | 4 (16·7%) | 2 (2·4%) | 6 (5·7%) | 0 | 0 | 0 | 72 (75·8%) | 10 (7·9%) | 82 (36·9%) | |
| Blood type | ||||||||||
| O | 8 (33·3%) | 31 (37·8%) | 39 (36·8%) | 11 (35·5%) | 2 (8·0%) | 13 (23·2%) | 34 (35·8%) | 36 (28·3%) | 70 (31·5%) | |
| A | 12 (50·0%) | 34 (41·5%) | 46 (43·4%) | 12 (38·7%) | 14 (56·0%) | 26 (46·4%) | 40 (42·1%) | 62 (48·8%) | 102 (45·9%) | |
| B | 1 (4·2%) | 11 (13·4%) | 12 (11·3%) | 5 (16·1%) | 3 (12·0%) | 8 (14·3%) | 12 (12·6%) | 14 (11·0%) | 26 (11·7%) | |
| AB | 3 (12·5%) | 6 (7·3%) | 9 (8·5%) | 3 (9·7%) | 3 (12·0%) | 6 (10·7%) | 8 (8·4%) | 8 (6·3%) | 16 (7·2%) | |
| Unknown | 0 | 0 | 0 | 0 | 3 (12·0%) | 3 (5·4%) | 1 (1·1%) | 7 (5·5%) | 8 (3·6%) | |
| Positive blood Rhesus factor | 20 (83·3%) | 74 (90·2%) | 94 (88·7%) | 30 (96·8%) | 22 (88·0%) | 52 (92·9%) | 89 (93·7%) | 111 (87·4%) | 200 (90·1%) | |
| Chronic disease | 9 (37·5%) | 66 (80·5%) | 75 (70·8%) | 20 (64·5%) | 15 (60·0%) | 35 (62·5%) | 35 (36·8%) | 101 (79·5%) | 136 (61·3%) | |
| Autoimmune disease | 3 (12·5%) | 22 (26·8%) | 25 (23·6%) | 10 (32·3%) | 5 (20·0%) | 15 (26·8%) | 14 (14·7%) | 36 (28·3%) | 50 (22·5%) | |
| Thyroid disease | 2 (8·3%) | 17 (20·7%) | 19 (17·9%) | 9 (29·0%) | 4 (16·0%) | 13 (23·2%) | 11 (11·6%) | 33 (26·0%) | 44 (19·8%) | |
| Arthritis | 0 | 4 (4·9%) | 4 (3·8%) | 1 (3·2%) | 0 | 1 (1·8%) | 1 (1·1%) | 1 (0·8%) | 2 (0·9%) | |
| Cardiovascular disease | 0 | 24 (29·3%) | 24 (22·6%) | 3 (9·7%) | 8 (32·0%) | 11 (19·6%) | 3 (3·2%) | 44 (34·6%) | 47 (21·2%) | |
| Hypertension | 3 (12·5%) | 42 (51·2%) | 45 (42·5%) | 9 (29·0%) | 8 (32·0%) | 17 (30·4%) | 11 (11·6%) | 64 (50·4%) | 75 (33·8%) | |
| Chronic obstructive pulmonary disease | 1 (4·2%) | 4 (4·9%) | 5 (4·7%) | 4 (12·9%) | 1 (4·0%) | 5 (8·9%) | 2 (2·1%) | 4 (3·1%) | 6 (2·7%) | |
| Chronic kidney disease | 0 | 4 (4·9%) | 4 (3·8%) | 0 | 0 | 0 | 1 (1·1%) | 1 (0·8%) | 2 (0·9%) | |
| Diabetes | 2 (8·3%) | 22 (26·8%) | 24 (22·6%) | 3 (9·7%) | 3 (12·0%) | 6 (10·7%) | 8 (8·4%) | 40 (31·5%) | 48 (21·6%) | |
| Cancer | 1 (4·2%) | 3 (3·7%) | 4 (3·8%) | 0 | 2 (8·0%) | 2 (3·6%) | 1 (1·1%) | 4 (3·1%) | 5 (2·3%) | |
| Use of immunosuppression, chemotherapy, or corticosteroids | 0 | 0 | 0 | 0 | 1 (4·0%) | 1 (1·8%) | 2 (2·1%) | 3 (2·4%) | 5 (2·3%) | |
| Thalassaemia | 0 | 0 | 0 | 0 | 0 | 0 | 6 (6·3%) | 0 | 6 (2·7%) | |
| Allergies | 6 (25·0%) | 8 (9·8%) | 14 (13·2%) | 5 (16·1%) | 5 (20·0%) | 10 (17·9%) | 8 (8·4%) | 21 (16·5%) | 29 (13·1%) | |
| Pregnancy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Smoker | 4 (16·7%) | 11 (13·4%) | 15 (14·2%) | 9 (29·0%) | 8 (32·0%) | 17 (30·4%) | 36 (37·9%) | 8 (6·3%) | 44 (19·8%) | |
| Physical health | ||||||||||
| Fair | 0 | 9 (11·0%) | 9 (8·5%) | 5 (16·1%) | 4 (16·0%) | 9 (16·1%) | 5 (5·3%) | 11 (8·7%) | 16 (7·2%) | |
| Good | 6 (25·0%) | 43 (52·4%) | 49 (46·2%) | 16 (51·6%) | 11 (44·0%) | 27 (48·2%) | 38 (40·0%) | 71 (55·9%) | 109 (49·1%) | |
| Very good | 15 (62·5%) | 16 (19·5%) | 31 (29·2%) | 8 (25·8%) | 9 (36·0%) | 17 (30·4%) | 27 (28·4%) | 39 (30·7%) | 66 (29·7%) | |
| Excellent | 3 (12·5%) | 7 (8·5%) | 10 (9·4%) | 2 (6·5%) | 1 (4·0%) | 3 (5·4%) | 3 (3·2%) | 2 (1·6%) | 5 (2·3%) | |
| Mental health | ||||||||||
| Fair | 1 (4·2%) | 6 (7·3%) | 7 (6·6%) | 1 (3·2%) | 0 | 1 (1·8%) | 1 (1·1%) | 3 (2·4%) | 4 (1·8%) | |
| Good | 6 (25·0%) | 21 (25·6%) | 27 (25·5%) | 12 (38·7%) | 9 (36·0%) | 21 (37·5%) | 23 (24·2%) | 42 (33·1%) | 65 (29·3%) | |
| Very good | 9 (37·5%) | 28 (34·1%) | 37 (34·9%) | 12 (38·7%) | 14 (56·0%) | 26 (46·4%) | 33 (34·7%) | 56 (44·1%) | 89 (40·1%) | |
| Excellent | 8 (33·3%) | 20 (24·4%) | 28 (26·4%) | 6 (19·4%) | 2 (8·0%) | 8 (14·3%) | 16 (16·8%) | 22 (17·3%) | 38 (17·1%) | |
Data presented as n (%) or median (IQR).
The rate of any solicited vaccination reaction was 50·0% in CoronaVac after any dose, 77·4% in BNT162b2 after any dose, and 94·6% in ChAdOx1 after the first dose (because of the differential timing of survey administration). In the CoronaVac group, a similar rate of local (32·4%) and systemic (30·6%) reactions were observed. A higher rate of local reactions (62·3%) was reported in the BNT162b2 group compared with systemic reactions (45·3%). ChAdOx1 had the highest rates of reactogenicity reported after the first dose, with 89·3% reporting systemic and 82·1% reporting local reactogenicity. In all vaccine groups, most frequently reported local reaction was pain, and most frequently reported systemic reactions were malaise and headache. In all vaccine groups, the rates of any local or systemic reaction appeared higher in the younger age group (<60 years) compared with the older age group (≥60 years; table 2 ).
Table 2.
Solicited vaccination reactions by vaccine and age groups
|
BNT162b2 |
ChAdOx1 |
CoronaVac |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age <60 years (n=24) | Age ≥60 years (n=82) | All (n=106) | Age <60 years (n=31) | Age ≥60 years (n=25) | All (n=56) | Age <60 years (n=95) | Age ≥60 years (n=127) | All (n=222) | ||
| Any reaction | 22 (91·7%) | 60 (73·2%) | 82 (77·4%) | 31 (100·0%) | 22 (88·0%) | 53 (94·6%) | 62 (65·3%) | 49 (38·6%) | 111 (50·0%) | |
| Local reaction | 21 (87·5%) | 45 (54·9%) | 66 (62·3%%) | 26 (83·9%) | 20 (80·0%) | 46 (82·1%) | 48 (50·5%) | 24 (18·9%) | 72 (32·4%) | |
| Pain | 19 (79·2%) | 39 (47·6%) | 58 (54·7%) | 24 (77·4%) | 20 (80·0%) | 44 (78·6%) | 46 (48·4%) | 21 (16·5%) | 67 (30·2%) | |
| Induration | 9 (37·5%) | 14 (17·1%) | 23 (21·7%) | 11 (35·5%) | 7 (28·0%) | 18 (32·1%) | 10 (10·5%) | 6 (10·5%) | 16 (7·2%) | |
| Redness | 2 (8·3%) | 6 (7·3%) | 8 (7·5%) | 1 (3·2%) | 2 (8·0%) | 3 (5·4%) | 2 (2·1%) | 1 (0·8%) | 3 (1·4%) | |
| Systemic reaction | 14 (58·3%) | 34 (41·5%) | 48 (45·3%) | 30 (96·8%) | 20 (80·0%) | 50 (89·3%) | 34 (41·5%) | 34 (26·8%) | 68 (30·6%) | |
| Malaise | 9 (37·5%) | 24 (29·3%) | 33 (31·1%) | 26 (83·9%) | 14 (56·0%) | 40 (71·4%) | 21 (22·1%) | 15 (11·8%) | 36 (16·2%) | |
| Headache | 6 (25·0%) | 14 (17·1%) | 20 (18·9%) | 22 (71·0%) | 13 (52·0%) | 35 (62·5%) | 21 (22·1%) | 14 (11·0%) | 35 (15·8%) | |
| Myalgia | 7 (29·2%) | 12 (14·6%) | 19 (17·9%) | 22 (71·0%) | 11 (44·0%) | 33 (58·9%) | 9 (9·5%) | 2 (1·6%) | 11 (5·0%) | |
| Joint pain | 5 (20·8%) | 7 (8·5%) | 12 (11·3%) | 20 (64·5%) | 11 (44·0%) | 31 (55·4%) | 8 (8·4%) | 2 (1·6%) | 10 (4·5%) | |
| Fever and shivering | 4 (16·7%) | 9 (11·0%) | 13 (12·3%) | 20 (64·5%) | 9 (36·0%) | 29 (51·8%) | 4 (4·2%) | 6 (4·7%) | 10 (4·5%) | |
| Shivering | 4 (16·7%) | 7 (8·5%) | 11 (10·4%) | 16 (51·6%) | 9 (36·0%) | 25 (44·6%) | 4 (4·2%) | 6 (4·7%) | 10 (4·5%) | |
| Fever | 1 (4·2%) | 5 (6·1%) | 6 (5·7%) | 14 (45·2%) | 6 (24·0%) | 20 (35·7%) | 1 (1·1%) | 0 | 1 (0·5%) | |
| Nausea | 1 (4·2%) | 7 (8·5%) | 8 (7·5%) | 8 (25·8%) | 5 (20·0%) | 13 (23·2%) | 5 (5·3%) | 3 (2·4%) | 8 (3·6%) | |
| Vomiting | 0 | 0 | 0 | 2 (6·5%) | 0 | 2 (3·6%) | 4 (4·2%) | 1 (0·8%) | 5 (2·3%) | |
| Dizziness | 2 (8·3%) | 3 (3·7%) | 5 (4·7%) | 6 (19·4%) | 4 (16·0%) | 10 (17·9%) | 4 (4·2%) | 4 (3·1%) | 8 (3·6%) | |
| Diarrhoea | 0 | 1 (1·2%) | 1 (0·9%) | 2 (6·5%) | 2 (8·0%) | 4 (7·1%) | 3 (3·2%) | 2 (1·6%) | 5 (2·3%) | |
| Stomach-ache | 0 | 0 | 0 | 3 (9·7%) | 1 (4·0%) | 4 (7·1%) | 3 (3·2%) | 0 | 3 (1·4%) | |
Data presented as n (%). Summary includes vaccination reactions reported after the first or second dose of vaccination in the BNT162b2 and CoronaVac groups, and reactions reported only after the first dose of vaccination in the ChADOx1 group because of the differential timing of survey administrations.
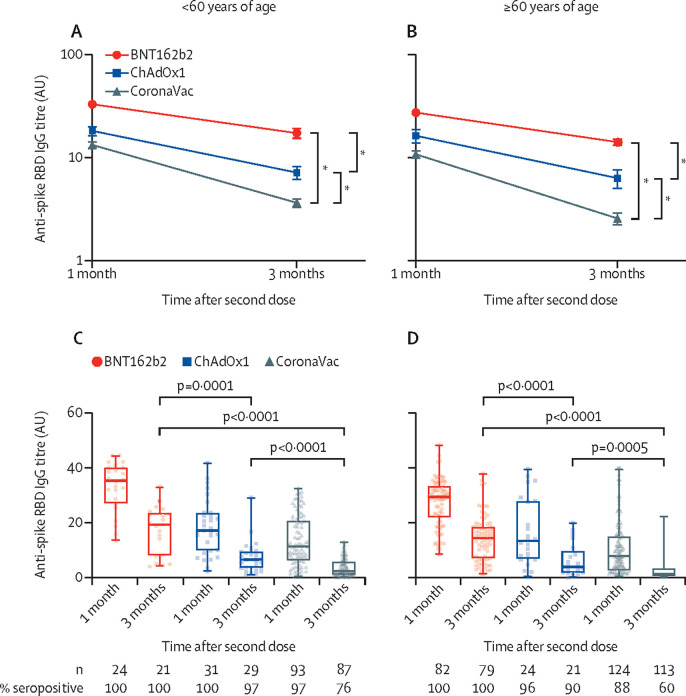
Assessment of anti-spike protein RBD IgG antibody titres at 1 month after the second dose of BNT162b2 revealed 100% seropositivity rates for both younger (<60 years) and older (≥60 years) age groups and stayed at 100% at 3 months after the second dose for both groups. In the ChAdOx1 group, seropositivity rates at 1 month after the second dose were 100% in the younger and 96% in the older age groups, which were reduced to 97% in the younger and 90% in the older age groups at 3 months after the second dose. In the CoronaVac group, seropositivity rates were 97% in the younger and 88% in the older age groups at 1 month after the second dose, and these rates dropped to 76% in the younger and 60% in the older age groups at 3 months after the second dose. In the younger age group, anti-spike RBD IgG antibody concentrations at 1 month after the second dose were highest with BNT162b2 (median 35·3 [IQR 27·6–40·0], followed by ChAdOx1 (17·1 [9·9–23·6]), and lowest in the CoronaVac group (11·3 [6·2–20·7]). At 3 months, the corresponding median antibody titres were 19·2 (8·2–23·1) in BNT162b2, 6·5 (3·5–9·3) in ChAdOx1, and 2·4 (1·0–5·0) in CoronaVac (p<0·0001 for both timepoints; figure 1A and C ). Similar trends were observed in the older age group, with the median anti-spike RBD IgG antibody titres at 1 month after the second dose being 29·4 (22·5–33·3) for BNT162b2, 13·3 (6·9–27·7) for ChAdOx1, and 6·4 (2·5–13·6) for CoronaVac, and at 3 months, 14·8 (7·4–18·7) for BNT162b2, 3·9 (1·9–8·4) for ChAdOx1, and 1·3 (0·5–3·3) for CoronaVac (p<0·0001 for both timepoints; figure 1B and D).
Figure 1.
Anti-spike RBD IgG titres by age group and vaccine type
(A) Mean (standard error) anti-spike RBD IgG titres in individuals aged younger than 60 years at 1 and 3 months after the second vaccination dose of BNT162b2, ChAdOx1, or CoronaVac vaccines. (B) Mean (SE) anti-spike RBD IgG titres in individuals aged 60 years or older at 1 and 3 months after the second vaccination dose of BNT162b2, ChAdOx1, or CoronaVac vaccines. (C) Box-and-whisker plots overlaid with individual anti-spike RBD IgG titres in individuals younger than 60 years at 1 and 3 months after the second vaccination dose of BNT162b2, ChAdOx1, or CoronaVac vaccines. (D) Box-and-whisker plots overlaid with individual anti-spike RBD IgG titres in individuals 60 years or older at 1 and 3 months after the second vaccination dose of BNT162b2, ChAdOx1, or CoronaVac vaccines. RBD=receptor binding domain. *p<0·0001 for both overall antibody titre during the 3-month follow-up period and the rate of decline over time from the multivariate linear repeated measures model (table 3).
In univariate linear repeated measures models, being male, older age, blood type of A (compared with type O), being overweight or obese (BMI ≥25), having a chronic disease, cardiovascular disease, and diabetes were each significantly (and hypertension marginally) associated with lower antibody titres. In the final linear multivariate repeated measures model, vaccine group (<0·0001) and vaccine group-by-time interaction (p<0·0001) were both significant; male sex (p=0·011), older age (<0·0001), and a longer follow-up time (p<0·0001) were significantly associated with lower antibody titres. Blood type, having a BMI of more than 25, and the aforementioned comorbidities lost their significance in the multivariate model. BNT162b2 was the vaccine group associated with a significantly higher overall antibody titre and with a significantly lower decline over time, followed by ChAdOx1, and finally CoronaVac (figure 1A, B; table 3 ). There was no significant difference in the rate of decline over time between the two age groups per vaccine type.
Table 3.
Linear repeated measures analysis of anti-spike receptor binding domain IgG antibody concentrations (log10) after the second dose of SARS-CoV-2 vaccination
| Estimate (95% CI) | p value | |
|---|---|---|
| Vaccine group | ||
| BNT162b2 | 0·57 (0·47 to 0·66) | <0·0001 |
| ChAdOx1 | 0·24 (0·12 to 0·36) | <0·0001 |
| CoronaVac (ref) | NA | NA |
| Male | −0·11 (−0·19 to −0·03) | 0·011 |
| Age (per 10 years) | −0·09 (−0·12 to −0·06) | <0·0001 |
| Time since second dose (per month) | −0·31 (−0·32 to −0·29) | <0·0001 |
| Vaccine group by time since second dose (per month) | ||
| BNT162b2 | 0·14 (0·11 to 0·16) | <0·0001 |
| ChAdOx1 | 0·08 (0·05 to 0·11) | <0·0001 |
| CoronaVac (ref) | NA | NA |
Within-person correlation of responses was taken into account via use of an unstructured covariance matrix. CoronaVac was used as the reference group in the models because it had the largest sample size among the three vaccine groups. NA=not applicable.
Collectively, these data show that in both age groups, BNT162b2 induced the highest overall anti-spike RBD IgG antibody titres, followed by ChAdOx1, and then by CoronaVac. In addition, the rate of decline of antibodies was fastest with CoronaVac, followed by ChAdOx1, and then by BNT162b2.
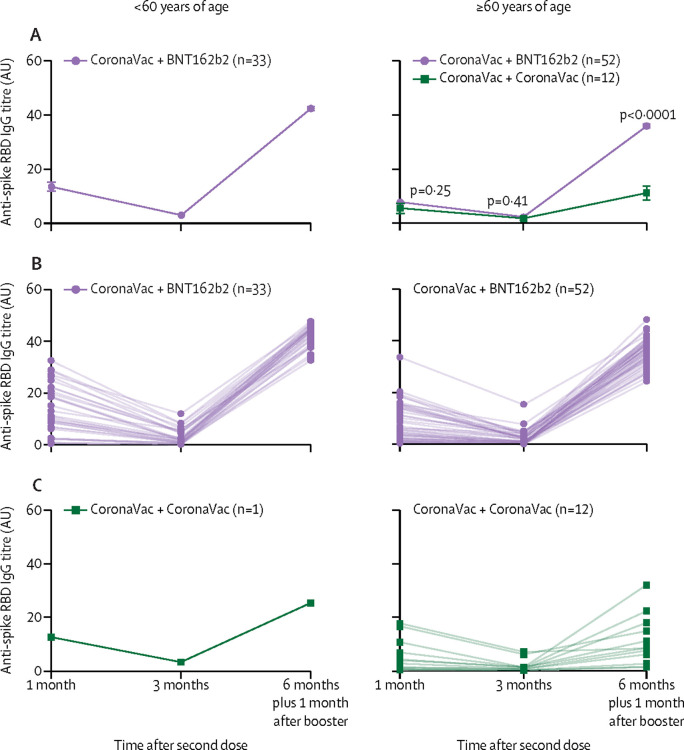
A cohort of 85 participants who initially received two doses of CoronaVac followed by a single boost of BNT162b2 at approximately 6 months after the primary vaccination dose was evaluated, of which 70 responded to the survey. Solicited vaccination reactions after the booster dose of BNT162b2 were similar to those after primary vaccination by BNT162b2 per age group, and no severe events or admissions to hospital were reported (table 4 ). 33 participants were <60 years, with a median age of 33·0 (IQR 27·0–44·0) and 60·6% were female. In this younger age group, median anti-spike RBD IgG titres were 11·0 (6·7–20·7) at 1 month and 2·1 (0·8–4·9) at 3 months, with seropositivity rates of 90·9% at 1 month and 66·7% at 3 months. After a single booster dose of BNT162b2, antibody titres increased significantly in all participants to a median titre of 44·0 (40·4–44·9; male participants, 43·1 [41·7–44·5]; female participants, 44·4 [39·2–45·0]), with a 100% response rate (geometric mean fold rise from 1 month after the second dose to 1 month after the booster of 4·7 [95% CI 3·2–6·9]). In 52 participants aged 60 years or older, the median age was 71·5 (IQR 66·0–77·0) years and 59·6% were female. In this older age group, the median antibody titres were 5·0 (1·8–13·9) at 1 month and 1·2 (0·5–3·1) at 3 months, with seropositivity rates of 86·5% at 1 month and 59·6% at 3 months. Similar to the younger age group, a single booster dose of BNT162b2 at 6 months after the first dose of CoronaVac generated significantly escalated antibody titres, with a median titre of 36·7 (33·0–39·3; male participants, 35·4 [31·0–39·6]; female participants, 36·8 [33·1–39·1]) and a 100% response rate, but with a more pronounced fold-increase compared with 1 month after the second dose titre (geometric mean fold rise 7·9 [95% CI 5·8–10·8]; p=0·024, Wilcoxon two-sample test for comparison of fold rise between the two age groups; figure 2A, B ).
Table 4.
Solicited vaccination reactions after a booster dose of BNT162b2 after the initial receipt of a complete CoronaVac regimen
| Age <60 years (n=28) | Age ≥60 years (n=42) | All (N=70*) | ||
|---|---|---|---|---|
| Any reaction | 25 (89·3%) | 31 (73·8%) | 56 (80·0%) | |
| Local reaction | 24 (85·7%) | 27 (64·3%) | 51 (72·9%) | |
| Pain | 24 (85·7%) | 25 (59·5%) | 49 (70·0%) | |
| Induration | 6 (21·4%) | 9 (21·4%) | 15 (21·4%) | |
| Redness | 0 | 3 (7·1%) | 3 (4·3%) | |
| Systemic reaction | 17 (60·7%) | 12 (28·6%) | 29 (41·4%) | |
| Malaise | 12 (42·9%) | 7 (16·7%) | 19 (27·1%) | |
| Headache | 12 (42·9%) | 7 (16·7%) | 19 (27·1%) | |
| Myalgia | 9 (32·1%) | 0 | 9 (12·9%) | |
| Joint pain | 10 (35·7%) | 0 | 10 (14·3%) | |
| Fever and shivering | 7 (25·0%) | 0 | 7 (10·0%) | |
| Shivering | 3 (10·7%) | 0 | 3 (4·3%) | |
| Fever | 6 (21·4%) | 0 | 6 (8·6%) | |
| Nausea | 3 (10·7%) | 0 | 3 (4·3%) | |
| Vomiting | 1 (3·6%) | 0 | 1 (1·4%) | |
| Dizziness | 4 (14·3%) | 0 | 4 (5·7%) | |
| Diarrhoea | 0 | 0 | 0 | |
| Stomach-ache | 2 (7·1%) | 0 | 2 (2·9%) | |
Data presented as n (%).
Of the 85 participants evaluated in this group, 70 responded to the survey after the booster dose.
Figure 2.
Anti-Spike RBD IgG titres by age group and vaccine plus booster
(A) Mean (SE) anti-spike RBD IgG titres in individuals younger than 60 years (left) or 60 years or older (right) at 1 and 3 months after the second CoronaVac vaccination dose, followed by 1 month after a BNT162b2 or CoronaVac booster shot given at 6 months after the initial CoronaVac vaccination. For the 60 years and older age group, the geometric mean fold rise in antibody titres from 1 month after baseline to after the booster was approximately 8-fold in the BNT162b2 boost group versus approximately 3-fold in the CoronaVac group (7·9 [95% CI 5·8–10·8] in the BNT162b2 boost group vs 2·8 [95% CI 1·6–5·0] in the CoronaVac boost group). p values comparing the two groups in the right panel are from the Wilcoxon two-sample test. (B) Individual anti-spike RBD IgG titres over time in individuals younger than 60 years (left) or 60 years or older (right) at 1 and 3 months after the second CoronaVac vaccination dose, followed by 1 month after a single BNT162b2 booster shot given at 6 months after the initial CoronaVac vaccination. (C) Individual anti-spike RBD IgG titres in individuals younger than 60 years (left) or 60 years or older (right) at 1 and 3 months after the second CoronaVac vaccination dose, followed by 1 month after a single CoronaVac booster shot given at 6 months after the initial CoronaVac vaccination. Only one individual younger than 60 years of age received CoronaVac as a booster shot, and thus has been omitted from the mean plot in panel A. RBD=receptor binding domain.
A small cohort of 13 participants who initially received two doses of CoronaVac and chose to receive another booster dose of CoronaVac at 6 months after the first dose was evaluated. 12 of the 13 participants were in the 60 years or older age group. In this older age group, the median age was 67·0 (IQR 64·5–74·0) and 50·0% were female. Median antibody titres were 3·2 (0·9–8·6) for 1 month and 0·9 (0·4–1·3) for 3 months, with seropositivity rates of 75% at 1 month and 50% at 3 months. After receipt of a single booster dose of CoronaVac, antibody titres increased to a median titre of 8·4 (4·3–16·3; male participants, 8·4 [2·6–17·9]; female participants, 9·3 [6·0–14·8]; 100% response), which reflected a more modest fold-increase relative to titres at 1 month after the second dose (geometric mean fold rise 2·8 [95% CI 1·6–5·0]; figure 2A, C).
Collectively, although the IgG titres at 1 month and 3 months after the second dose of CoronaVac were similar between the two older age groups (Wilcoxon two-sample test; p=0·25 at 1 month and p=0·41 at 3 months), a single booster dose of BNT162b2 induced higher anti-spike RBD IgG antibody titres compared with a single booster dose of CoronaVac (p<0·0001; figure 2A).
We evaluated an independent small reference group of 29 participants (62% female, median age 43 [IQR 35–54]) who recovered from a natural SARS-CoV-2 infection in the past 3 months. Timing of the first assessment was on average 2 months after a negative PCR post-infection, and the median antibody titres were 25·9 (IQR 17·2–34·6) for two participants requiring oxygen therapy or intensive care unit support, or both; 14·8 (12·4–17·5) for six participants requiring admission to hospital but without critical disease; and 5·0 (2·4–9·0) for 21 participants who had mild disease. 19 individuals had a second assessment approximately 2 months after their initial assessment: the antibody titre for one participant requiring oxygen therapy went down from 34·6 to 29·3; the median titre for four other participants in hospital decreased from 16·6 (14·8–20·9) to 10·1 (6·0–14·6; median fold change, 0·52 [0·41–0·71]); and the median titre for mild cases was lowered from 2·9 (2·4–8·3) to 2·5 (1·7–5·0; median fold change, 0·83 [0·63–0·99]). Relative to the three vaccine groups, a more modest average decline was observed over time in individuals who recovered from natural infection (appendix).
During the study follow-up to date, six participants (four male participants; median age of 46; range 33–73) were diagnosed with COVID-19 post-study enrolment. Five were in the CoronaVac and one in the ChAdOx1 group; all had antibody titres less than 10 at their last visit before diagnosis (0·5, 1·0, 4·7, 5·6, 7·5, and 7·8). Three cases were diagnosed in between their 1 month and 3 month visits; three were diagnosed after their 3 month visit. One male participant aged 64 years, in the ChAdOx1 group with chronic conditions had to be accepted into the intensive care unit but has recovered. One female participant aged 73 years, in the CoronaVac group was followed up in the hospital for precaution. There were no other admissions to hospital or oxygen therapy requirements among the participants.
Discussion
Vaccines have been shown to reduce COVID-19 severity in a safe and effective manner. Establishing how long this protection will last for different vaccines is an ongoing challenge. We have longitudinally compared SARS-CoV-2 anti-spike RBD IgG antibody responses to CoronaVac, ChAdOx1, and BNT162b2 vaccination, and in addition evaluated single booster doses by BNT162b2 or CoronaVac after a full CoronaVac regimen. The main findings of this study are first, that a higher rate of seropositivity and titre of antibodies were observed at all timepoints tested for BNT162b2 and ChAdOx1 vaccines compared with CoronaVac for all age groups, and that BNT162b2-induced IgG titres were also higher when compared with ChAdOx1. For the older age group at a high risk, the rate of seropositivity at 3 months after the second dose was 100% for BNT162b2, 90% for ChAdOx1, and 60% for CoronaVac. Second, regardless of age, the rate of decline of antibodies from 1 to 3 months after complete vaccination was fastest in the CoronaVac group, followed by ChAdOx1, and then by BNT162b2. Third, for older individuals vaccinated primarily with CoronaVac, a single booster dose of BNT162b2 led to significantly higher IgG titres when compared with a single booster dose of CoronaVac. To our knowledge, this is the first study designed to prospectively compare these three primary vaccines head-to-head in a longitudinal manner and can contribute to vaccine policy decisions. Our results are consistent with previous studies highlighting male sex and older age as being associated with lower overall antibody titres,16, 17, 18 where all tested vaccines led to lower titres of anti-spike RBD IgG antibody in male participants and older individuals.
Anti-spike RBD IgG antibodies bind to the RBD of SARS-CoV-2 and might inhibit viral attachment and entry into the host cell, thus suppressing infection. Although the variables and threshold of humoral response correlating with protection are not yet detailed, studies point to a strong relationship between high titres of anti-spike IgG and a reduced disease state as well as a lower occurrence of breakthrough infections, where higher IgG binding led to greater protection.13, 19 Lower anti-spike RBD IgG antibody concentrations are correlated with a higher risk of infection in individuals vaccinated with mRNA-1273 (Moderna).20 Furthermore, a strong correlation observed between anti-spike RBD IgG titres and neutralising antibody titres suggests that IgG concentrations offer indications on protection.21 Low anti-spike antibodies have been shown to predict mortality and viral dissemination in patients with COVID-19 who are critically ill.22 Although more data are required to understand the dynamics of antibody concentrations and cellular responses, antibody titres can serve as a correlate of protection against infection and a surrogate of general immune response to a specific vaccine.
Although vaccines confer protection against severe disease and admission to hospital,23, 24 waning immunity over several months25 along with the emergence of highly transmissible SARS-CoV-2 variants have prompted the scientific community to investigate the potential of booster doses. Current studies highlight the efficient neutralisation capacity of a third BNT162b2 dose after a primary two-dose BNT162b2 regimen26 as well as the safety and immunogenicity of a third dose of the ChAdOx1 vaccine.27 Studies evaluating the heterologous boost regimens of BNT162b2 and ChAdOx1 showed stronger T-cell responses,28 increased neutralisation,29 and improved reactogenicity.30
Here we report for the first time the differential increase in anti-spike RBD IgG titres upon boosting with BNT162b2 versus CoronaVac approximately 6 months after a full CoronaVac regimen. In both age groups, a third dose of BNT162b2 after a full regimen of CoronaVac gave rise to a significant increase in anti-spike RBD IgG titres. A booster dose of CoronaVac administered 6 months after a primary CoronaVac regimen gave rise to a more modest increase in IgG titres compared with boosting with BNT162b2 (geometric mean fold rise of approximately 3-fold with CoronaVac vs 8-fold with BNT162b2). Our results indicate a significant amount of IgG stimulation upon boosting with BNT162b2 after primary vaccination with CoronaVac, which could potentially contribute to higher and longer protection.
The limitations of our study include using a single-nationality cohort, low sample size, and convenience sampling method used for efficient enrolment. The differences in age ranges for different vaccines are ascribed by the regulatory vaccine rollout schemes.
Future studies should investigate how cellular immunity is affected by different boosting regimens as well as neutralising antibody titres to different variants. This research can further be extended to different vaccines and boosting regimens currently in use. Our study has implications for policy change; for instance, higher and potentially longer antibody responses can be obtained if the mRNA vaccine (such as BNT162b2) booster doses are administered after inactivated vaccines such as CoronaVac. This finding also supports the use of mixed vaccine doses for boosters.
Data sharing
Individual, deidentified participant data that underlie the results reported in this article (text, tables, figures, and appendix) can be made available upon formal request submitted to the corresponding author.
Declaration of interests
BB is currently an employee of The Emmes Company. The Emmes Company did not fund the study, nor did it play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. ÖU is currently an employee of the Novartis Institutes for Biomedical Research. This study was done entirely independently from Novartis. Novartis did not request, authorise, or fund the study, nor did it play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views and opinions expressed in this publication are those of the authors and do not necessarily reflect the official policy or position of Novartis or any of its officers. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank all participants of the study. This work was supported in part by Etik Hospital, CCO Solar, Akçaba Communications, and JetGaz, as well as individual donations. These funds were used in purchasing test kits. We thank Etik Hospital Biochemistry Laboratory (Durdugül Özserbes, Hacı Mehmet Mızrak, and team), Dünya IVF clinic (Özgü Özün and team), Mikrolab Biochemistry Laboratory (Tüge Göktuğ), and Duolab Biochemistry Laboratory (Nur Durmazer) for contributing staff time and equipment and materials for use, and assistance in sample collection or testing, or both. We thank Sultan Seven Öğmen for her assistance in participant recruitment and sample collection in the field. We thank Hatice Büyükoğlu and Zişan Cürcani for their support in participant follow-up and survey administration via telephone.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
BB conceptualised and coordinated the study, enrolled participants, curated data, formally analysed the data, acquired funding, and wrote and edited the paper. UK enrolled participants, did the antibody measurements, input the data, and acquired funding. FS enrolled participants and did the data entries. EV conceptualised the study, enrolled participants, acquired funding, and wrote and edited the paper. ÖU conceptualised the study, enrolled participants, made the figures, acquired funding, and wrote and edited the paper.
Supplementary Material
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barin B, Yoldascan BE, Savaskan F, Ozbalikci G, Karaderi T, Çakal H. Joint investigation of 2-month post-diagnosis IgG antibody levels and psychological measures for assessing longer term multi-faceted recovery among COVID-19 cases in northern Cyprus. Front Public Health. 2021;8 doi: 10.3389/fpubh.2020.590096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu D, Zhang G, Wang Y, et al. Structural basis for SARS-CoV-2 neutralizing antibodies with novel binding epitopes. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkan E. COVID-19: structural considerations for virus pathogenesis, therapeutic strategies and vaccine design in the novel SARS-CoV-2 variants era. Mol Biotechnol. 2021;63:885–897. doi: 10.1007/s12033-021-00353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkan E, Volkan E. Under the COVID-19 lockdown: rapid review about the unique case of north Cyprus. Psychol Trauma. 2020;12:539–541. doi: 10.1037/tra0000809. [DOI] [PubMed] [Google Scholar]
- 15.Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against omicron or delta infection. medRxiv. 2022 doi: 10.1101/2021.12.30.21268565. published online Jan 1. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauré D, O'Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2022;22:56–63. doi: 10.1016/S1473-3099(21)00479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Vicente M, Almansa R, Martínez I, et al. Low anti-SARS-CoV-2 S antibody levels predict increased mortality and dissemination of viral components in the blood of critical COVID-19 patients. J Intern Med. 2021 doi: 10.1111/joim.13386. published online Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falsey AR, Frenck RW, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenbusch M, Schumacher S, Vogel E, et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021;21:1212–1213. doi: 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual, deidentified participant data that underlie the results reported in this article (text, tables, figures, and appendix) can be made available upon formal request submitted to the corresponding author.