Abstract
Lipomatosis of lymph nodes is defined as the replacement of the lymphatic parenchyma by adipose tissue which grows in the node from the hilus toward the cortical zone. In humans, it is considered as part of the normal aging process and is common in obese patients, but there are no reports in non-human primates. In this report, we describe the first case of lymph node lipomatosis in the bilateral axillary lymph nodes of a young adult cynomolgus monkey. Macroscopically, there were no apparent abnormalities in the axillary lymph nodes on either side, and their volumes were unchanged. At the cut surface, pale yellow fat-like tissue was observed in the medullary area. Histopathologically, well differentiated adipocytes replaced a large part of the lymphatic parenchyma in the area from the hilus to the medulla without any malignant findings. Based on these findings, the patient was diagnosed with lipomatosis of the lymph nodes.
Keywords: lipomatosis, adipocytes, axillary lymph node, cynomolgus monkey, spontaneous lesion
Lipomatosis of lymph nodes is the replacement of lymphatic parenchyma by adipose tissue which grows in the node from the hilus toward the cortical zone1, 2 and is regarded as a non-malignant change and a part of the normal aging process in humans3, 4. Lipomatosis of lymph nodes is also called fatty infiltration1, lipomatous atrophy2, 5, fatty replacement4, or fatty change5, 6. Lipomatosis has been observed in the axillary lymph nodes of aging mice7, but to our knowledge, there have been no reported cases in non-human primates. Here, we describe a case of spontaneously arising lipomatosis of the axillary lymph nodes in a cynomolgus monkey.
A six year old male cynomolgus monkey (Macaca fascicularis) of Cambodian origin was purchased from Shin Nippon Biomedical Laboratories, Ltd. Blood was sampled without any test article treatment. No remarkable findings were observed in the clinical observations or blood tests from routine medical examinations, and the final body weight was 6.5 kg. At necropsy, only involution of the thymus was observed. All animal procedures were conducted in accordance with the Chugai Pharmaceutical Guide for the Care and Use of Laboratory Animals, and all experimental protocols were approved by the Institutional Animal Care and Use Committee.
Macroscopically, there were no apparent abnormalities in the axillary lymph nodes on either side, and their volumes were unchanged. Therefore, all organs, including one axillary lymph node from each side, were fixed in 10% neutral-buffered formalin and embedded in paraffin for specimen preparation. After fixation, pale yellow fat-like tissue was observed in the medullary area at the cut surface of both the axillary lymph nodes (Fig. 1).
Fig. 1.

The macroscopic finding of axillary lymph node on one side. On the cut surface, a pale yellow fat-like tissue was present in medullary area. The nodal volume showed no change. Bar=2 mm
All tissue sections embedded in paraffin were stained with hematoxylin and eosin (HE), and the sections of the axillary lymph nodes were stained with azan stain using standard methods. Frozen sections were used for Oil Red O staining of the axillary lymph nodes. For immunohistochemical analysis8, the sections of the axillary lymph nodes were subjected to primary antibody against Ki-67 (SP6, rabbit monoclonal anti-Ki-67, Abcam, Cambridge, UK, ab16667) to analyze the proliferative activity of adipocytes. The sections were incubated with goat anti-rabbit secondary antibody (Dako, Glostrup, Denmark, E0432) followed by incubation with avidin-biotin-peroxidase complex (ABC peroxidase standard staining kit, Thermo Fisher Scientific, Waltham, MA, USA). The reaction was visualized using 3,3-diaminobenzidine tetrachloride. Finally, the sections were counterstained with Mayer’s hematoxylin.
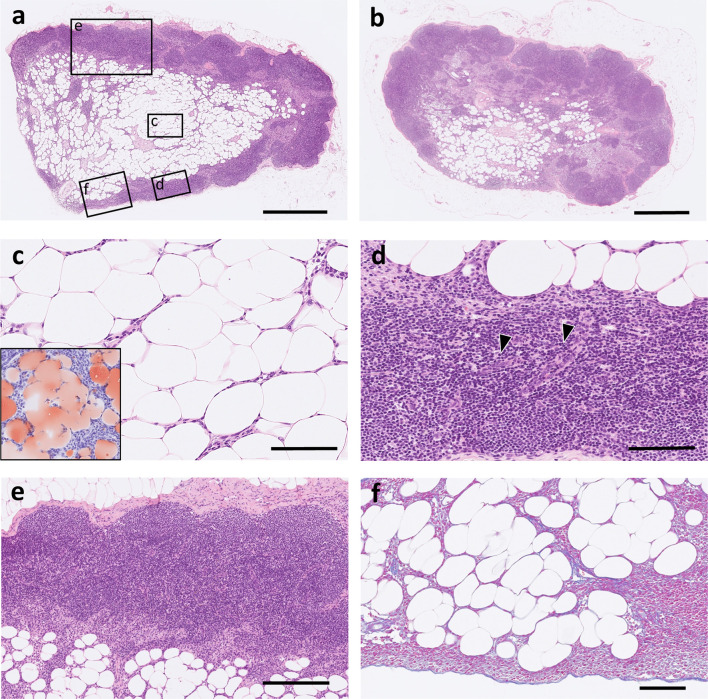
On microscopic examination, the medullary lymphatic parenchyma was replaced by adipose tissue-like structures, and the medullary area, including the hilus, expanded in both the axillary lymph nodes (Fig. 2a, 2b). Adipose tissue-like structures were composed of Oil Red O positive well-differentiated adipocytes, with a single large fat droplet in the cytoplasm and peripherally located nuclei (Fig. 2c). The adipose tissue that mainly occupied the area from the hilus to the medulla was focally located adjacent to the paracortical area, but the adipocytes did not show invasive growth and cellular atypia (Fig. 2d). There were few morphological changes in the cortical area, except for the vague formation of germinal centers (Fig. 2e). The periphery of the axillary lymph node was covered with a fibrous capsule, and intranodal adipocytes did not infiltrate the adjacent adipose tissue beyond the capsule (Fig. 2f). Immunohistochemically, Ki-67 expression was detected in lymphocytes of the lymphoid follicles, but the increased number of adipocytes were negative for Ki-67 (Fig. 3).
Fig. 2.
The microscopic findings of axillary lymph nodes (a) The histopathological image corresponds to the cut surface of Fig. 1. The adipose tissue replaced large part of normal medullary lymphatic parenchyma. The medullary area including hilus was expanded by adipose tissue. The circles on the figure with letters “c”, “d”, and “e” indicates the display region in Fig. 2c, 2d, and 2e, respectively. The area labeled “f” is equivalent to the Azan-stained area in Fig. 2f. HE stain. Bar=1 mm. (b) The adipose tissue was also observed in the medullary area of the contralateral axillary lymph node. HE stain. Bar=1 mm. (c) The intranodal adipose tissue was composed of well-differentiated adipocytes. HE stain. (inner box: Positive staining for Oil Red O demonstrated that these cells were adipocytes.) Bar=100 µm. (d) In a part of the lesion, the adipose tissue adjacent to the paracortical area without invasive growth and cellular atypia. Arrowheads point at high endothelial vein located in paracortical area. HE stain. Bar=100 µm. (e) Few morphological changes were seen in cortical area, although germinal center was obscure. HE stain. Bar=250 µm. (f) The axillary lymph node was covered with the fibrous capsule and intranodal adipocytes did not invade adjacent adipose tissue beyond the capsule. Azan stain. Bar=100 µm.
Fig. 3.
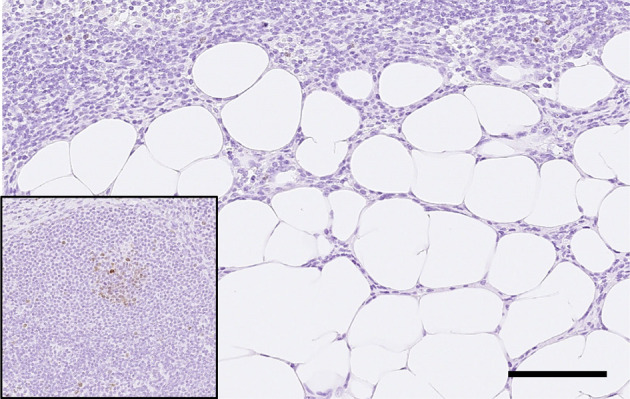
Intranodal adipocytes were negative for Ki-67 immunohistochemical staining. (inner box: Lymphocytes of the lymphoid follicle were positive for Ki-67.) Bar=100 µm.
In the present case, a large proportion of the medullary parenchyma of the bilateral axillary lymph nodes was occupied by adipose tissue without any malignant findings. This lesion was diagnosed as lipomatosis of the lymph nodes. To confirm whether lipomatosis appeared in other lymph nodes in this monkey, the mesenteric lymph node and pancreatic hilar lymph node were histopathologically examined. Lipomatosis was not observed in these lymph nodes (data not shown). There were no remarkable histopathological findings in other organs or tissues, except for some spontaneous changes.
The lesion in this case is a common finding in aged2, 5, 6 and obese patients5, 9 and is not a malignant change3, 4. It is especially common in peripheral lymph nodes that usually receive little antigen stimulation, such as cubital, axillary, and popliteal nodes2. Bilateral lipomatosis has been reported in the human axillary10, cervical, and inguinal lymph nodes3. Generally, in humans, adipose tissue replaces normal lymphatic parenchyma; therefore, nodal volume shows no change, but in some cases, lymph nodes may increase in volume3, 5. The axillary lymph nodes were not enlarged in the present case, but histopathologically, the adipose tissue replaced a large part of the medullary parenchyma. In cynomolgus monkeys, there are no previously reported lipomatosis cases; however, in a discussion among experts in experimental animal histopathology at an academic conference, a few specialists mentioned finding a similar lesion in the axillary lymph nodes of cynomolgus monkeys used in toxicity studies. They suggested that lipomatosis might be associated with aging in cynomolgus monkeys, although the incidence of similar lesions was low.
Considering the histopathological features of lymph node lipomatosis, a possible differential diagnosis can include lipomatous tumors such as well-differentiated liposarcoma11 and lipoma12. However, to our knowledge, there are no reports of primary lipomatous tumors arising in lymph nodes, and the metastatic spread of liposarcoma to regional lymph nodes has rarely been reported in humans13, 14. In the present case, lipoblasts and nuclear atypia of adipocytes that are typically found in well-differentiated liposarcoma11 were absent. In addition, a fibrous capsule surrounding proliferative adipocytes, which is one of the common findings in lipoma12, was not present. Intranodal adipocytes did not exhibit invasive growth and did not compress adjacent tissue. According to immunohistochemical analysis, the adipocytes were negative for Ki-67, suggesting that these cells lacked proliferative activity. Furthermore, there were no extra-lymphatic primary lipomatous tumors in the cynomolgus monkey. These findings indicate that this lesion is not a primary or metastatic lipomatous tumor.
The site of lesion formation in this case corresponds to the site specificity of lipomatosis of the lymph nodes in humans. This monkey was a healthy young adult15 and was slightly overweight16, which does not completely reflect the findings normally associated with the majority of human patients, such as aging and obesity. However, in humans, lipomatosis of the axillary, popliteal, and cubital lymph nodes can be seen, to some extent, during infancy and childhood, with a comparatively higher rate in axillary lymph nodes2. Similar to humans, lipomatosis of lymph nodes may occur in cynomolgus monkeys at an early age in a site-specific manner, particularly in regions that are rarely invaded by antigens, for example, axillary lymph nodes. The origin of adipocytes observed in lipomatosis was suspected to be the reticular cells in lymph nodes3, 17, although the relationship between adipocytes and reticular cells is still under discussion18, 19. In the present case, adipocytes existed completely inside the lymph node capsule, similar to previously reported human and rodent lipomatosis cases7, 20, which means that the original cells were present in the lymph nodes. Although the pathogenesis of lipomatosis of lymph nodes is poorly understood, reticular cells may be responsible for this lesion.
In conclusion, the lymphatic parenchyma of both the axillary lymph nodes was replaced by adipose tissue without any malignant findings in a young adult cynomolgus monkey, and this lesion was diagnosed as lipomatosis of the lymph nodes. This is the first reported case of lipomatosis of lymph nodes in a cynomolgus monkey, and it is unclear whether lipomatosis is associated with aging and obesity or whether it occurs in a site-specific manner in this species, as in humans. More cases must be investigated to fully understand the etiology of lipomatosis of the lymph nodes in cynomolgus monkeys.
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank Mr. Toshihiko Shiga, Dr. Hiroko Azabu, and Dr. Mio Hiramatsu for their scientific and skillful technical support.
References
- 1.Leborgne R, Leborgne F, Jr , and Leborgne JH. Soft-tissue radiography of axillary nodes with fatty infiltration. Radiology. 84: 513–515. 1965. [DOI] [PubMed] [Google Scholar]
- 2.Luscieti P, Hubschmid T, Cottier H, Hess MW, and Sobin LH. Human lymph node morphology as a function of age and site. J Clin Pathol. 33: 454–461. 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflieger H, Manzke E, Kurrle E, and Heymer B. Generalized lipomatosis of lymph nodes: a lymphographic problem in differentiating from malignancy. A case presentation. Lymphology. 12: 262–266. 1979. [PubMed] [Google Scholar]
- 4.Smith T. Fatty replacement of lymph nodes mimicking lymphoma relapse. Cancer. 58: 2686–2688. 1986. [DOI] [PubMed] [Google Scholar]
- 5.Giovagnorio F, Drudi FM, Fanelli G, Flecca D, and Francioso A. Fatty changes as a misleading factor in the evaluation with ultrasound of superficial lymph nodes. Ultrasound Med Biol. 31: 1017–1022. 2005. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi O, McCall JL, and Stringer MD. Does senescence affect lymph node number and morphology? A systematic review. ANZ J Surg. 83: 612–618. 2013. [DOI] [PubMed] [Google Scholar]
- 7.Sainte-Marie G, and Peng FS. Morphological anomalies associated with immunodeficiencies in the lymph nodes of aging mice. Lab Invest. 56: 598–610. 1987. [PubMed] [Google Scholar]
- 8.Moroki T, Matsuo S, Hatakeyama H, Hayashi S, Matsumoto I, Suzuki S, Kotera T, Kumagai K, and Ozaki K. Databases for technical aspects of immunohistochemistry: 2021 update. J Toxicol Pathol. 34: 161–180. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diflorio Alexander RM, Haider SJ, MacKenzie T, Goodrich ME, Weiss J, and Onega T. Correlation between obesity and fat-infiltrated axillary lymph nodes visualized on mammography. Br J Radiol. 91: 20170110. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen M, Christensen L, Jensen J, Thomsen JL, and Andersen JA. Axillary lymph node morphology in women with in situ breast carcinoma. An autopsy study. Virchows Arch A Pathol Anat Histopathol. 412: 347–355. 1988. [DOI] [PubMed] [Google Scholar]
- 11.Laurino L, Furlanetto A, Orvieto E, and Dei Tos AP. Well-differentiated liposarcoma (atypical lipomatous tumors). Semin Diagn Pathol. 18: 258–262. 2001. [PubMed] [Google Scholar]
- 12.Salam GA. Lipoma excision. Am Fam Physician. 65: 901–904. 2002. [PubMed] [Google Scholar]
- 13.Geethamani V, Savithri R, Suguna BV, and Niveditha SR. Cytomorphology of dedifferentiated liposarcoma of the subcutis of the upper back with axillary lymph node metastasis: a case report. Acta Cytol. 54: 333–336. 2010. [DOI] [PubMed] [Google Scholar]
- 14.Sá IB, Carvalho J, Silva R, Ferreira P, Matos-Lima L, and Taveira-Gomes A. Liposarcoma with lymph node spread: a case presentation and a systematic review of the literature. Eur Surg. 47: 94–100 2015. [Google Scholar]
- 15.Schillaci MA, Jones-Engel L, Lee BPY-H, Fuentes A, Aggimarangsee N, Engel GA, and Sutthipat T. Morphology and somatometric growth of long-tailed macaques (Macaca fascicularis fascicularis) in Singapore. Biol J Linn Soc Lond. 92: 675–694. 2007. [Google Scholar]
- 16.Choi K, Chang J, Lee MJ, Wang S, In K, Galano-Tan WC, Jun S, Cho K, Hwang YH, Kim SJ, and Park W. Reference values of hematology, biochemistry, and blood type in cynomolgus monkeys from cambodia origin. Lab Anim Res. 32: 46–55. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demchenko GA, Abdreshov SN, Akhmetbaeva NA, Makashev EK, Nurmakhanova BA, Balkhybekova AO, and Kalekeshov AM. The specifics of adrenergic innervation of lymph nodes from different body regions in young, mature, and old animals. Bull Exp Biol Med. 170: 283–287. 2021. [DOI] [PubMed] [Google Scholar]
- 18.Bénézech C, Mader E, Desanti G, Khan M, Nakamura K, White A, Ware CF, Anderson G, and Caamaño JH. Lymphotoxin-β receptor signaling through NF-κB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity. 37: 721–734. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SY, Bae H, Jeong SH, Park I, Cho H, Hong SP, Lee DH, Lee CK, Park JS, Suh SH, Choi J, Yang MJ, Jang JY, Onder L, Moon JH, Jeong HS, Adams RH, Kim JM, Ludewig B, Song JH, Lim DS, and Koh GY. YAP/TAZ direct commitment and maturation of lymph node fibroblastic reticular cells. Nat Commun. 11: 519. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadamitzky C, Spohr H, Debertin AS, Guddat S, Tsokos M, and Pabst R. Age-dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance? J Anat. 216: 556–562. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]