Abstract
Introduction
Vaccination is an essential intervention to curb the coronavirus disease 2019 (COVID-19) pandemic. This review aimed to estimate the pooled proportion of COVID-19 vaccine acceptance worldwide.
Methods
A systematic search of the MEDLINE (PubMed) database using “COVID-19,” “vaccine” and “acceptance” to obtain original research articles published between 2020 and July 2021. Only studies with full text and that were published in English were included. The Joanna Briggs Institute meta-analysis was used to assess the data quality. The meta-analysis was performed using generic inverse variance with a random-effects model using the Review Manager software.
Results
A total of 172 studies across 50 countries worldwide were included. Subgroup analyses were performed with regard to vaccine acceptance, regions, population, gender, vaccine effectiveness, and survey time. The pooled proportion of COVID-19 vaccine acceptance was 61% (95% CI: 59, 64). It was higher in Southeast Asia, among healthcare workers, in males, for vaccines with 95% effectiveness, and during the first survey.
Conclusion
COVID-19 vaccine acceptance needs to be increased to achieve herd immunity to protect the population from the disease. It is crucial to enhance public awareness of COVID-19 vaccination and improve access to vaccines.
Systematic Review Registration
PROSPERO 2021, identifier CRD42021268645.
Keywords: COVID-19, vaccine, acceptance, meta-analysis, prevalence
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a global health topic of concern. As of August 2021, nearly 216 million COVID-19 cases have been reported globally, with the cumulative number of deaths being just under 4.5 million (1). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, has changed over time. Change in the virus's properties may affect disease transmissibility, severity, and vaccine efficacy. The World Health Organization (WHO) has identified four SARS-CoV-2 variants of concern, namely alpha, beta, gamma, and delta, and five variants of interest, namely eta, iota, kappa, lambda, and mu (2).
In December 2020, the WHO approved the first vaccine, BNT162b2/COMIRNATY, which contains modified nucleoside mRNA that confers protection against COVID-19 (3). The United States Food and Drug Administration has granted emergency use authorization to use the COVID-19 vaccines and full approval for Pfizer vaccine to control the pandemic. The WHO has also recommended several vaccines for COVID-19, including COVISHIELD™, Janssen, Vaxzervria, Moderna, BIBP, CoronaVac, and AstraZeneca (3). The vaccines have the potential to create herd immunity without causing illness and complications (4). Herd immunity requires sufficient coverage and a large proportion of the population to be vaccinated.
However, the effectiveness of vaccination depends on the population's willingness to accept the vaccines. This urgent use of newly developed vaccines evokes a sense of vigilance in the general population. Many factors influence the population's acceptance of the vaccination, including risk perception of the disease, perception of vaccine safety and efficacy, public vaccination attitudes, past vaccination history, doctor's recommendations, costs, convenience, and sociodemographic characteristics (5).
Many studies have explored public perceptions of the COVID-19 vaccination program. The acceptance of COVID-19 vaccines varies among countries globally (6). Some studies showed a high level of vaccination acceptance (5, 7–12), while others reported an average level of acceptance (7, 8). An analysis of social media users indicated that of all participants involved, 36.4% in New York, 51.3% in London, 67.3% in São Paulo, 69.8% in Mumbai, and 76.8% in Beijing were willing to accept COVID-19 vaccines (9). An online survey in Arab countries reported that 83% of the study participants were hesitant to accept the vaccines because of their side effects, distrust in health care policies, the expedited production of vaccines, published studies, and vaccine-producing companies (10).
Determining the pooled estimated proportion of COVID-19 vaccination acceptance provides guidance to health authorities to prepare for an effective vaccination program. A successful and effective vaccination program can provide sufficient vaccination coverage in a population to achieve herd immunity and subsequently control the COVID-19 pandemic. Thus, this review aims to assess the estimated proportion of acceptance of the COVID-19 vaccines.
Methods
Study Design
A systematic review and meta-analysis of studies were conducted to assess the proportion of COVID-19 vaccination acceptance. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11) were followed to review articles of the studies. Ethics review and approval are not required for analyses of published data. This review was registered in PROSPERO 2021 (CRD42021268645).
Eligibility Criteria
The criteria for inclusion include studies that report the proportion of COVID-19 vaccination acceptance. The acceptance of the vaccines included studies before or after the availability of COVID-19 vaccines. All types of COVID-19 vaccines were included in this review. Studies with cross-sectional, case-control, and cohort designs published in English from 2020 to 19 July 2021 were included. Case series/reports, conference papers, proceedings, articles available only in abstract form, editorial reviews, letters of communications, commentaries, systematic reviews, and qualitative studies were excluded. Articles in languages other than English were also be excluded.
Information Sources
A systematic search was performed in the MEDLINE (PubMed) database for articles between 1 January 2020 and 19 July 2021.
Search Strategy
The search was done using the generic free-text search terms “COVID-19” AND “vaccine” AND “acceptance.” All studies published from 2020 to 19 July 2021 were retrieved to assess their eligibility for inclusion in this study. The search was restricted to full-text only and English language articles. To find additional potentially eligible studies, reference lists of included citations were cross-checked.
Selection Process
All records identified by our search strategy were exported to EndNote software. Duplicate articles were removed from the list. One independent reviewer screened the titles and abstracts of the identified articles. The full texts of eligible studies were obtained and read thoroughly to assess their suitability. The second reviewer validated the records. A third reviewer was consulted in the event of a conflict between the two reviewers. The search method was presented in the PRISMA flow chart showing the included studies and excluded with reasons for exclusion.
Data Collection Process and Data Items
The data was extracted into Microsoft Excel (Microsoft Office Professional Plus 2016). The data included the first author, year of publication, study location, study design, setting, study population, sample size, proportion, and data to calculate effect estimates.
Study Risk of Bias Assessment
Assessment of critical appraisal for data quality was assessed using the Joanna Briggs Institute (J.B.I.). Meta-Analysis for cross-sectional, case-control, and cohort studies (12). Two authors performed bias assessments independently.
Effect Measures
The proportion of vaccines acceptance was reported in pooled estimate proportion with a 95% confidence interval. Terminology of vaccines acceptance refers to the willingness to be vaccinated, vaccines acceptability, desirability, demand, and positive attitudes toward the given vaccines.
Synthesis Methods
The analysis was performed with Review Manager (RevMan) software (13). A generic inverse variance with a random-effects model was applied to pool the proportion of the studie's data. The heterogeneity was assessed by I2 statistic and used the guide as outlined: 0% to 40% might not be important; 30 to 60% may represent moderate heterogeneity; 50 to 90% may represent substantial heterogeneity; and 75 to 100% would be considerable heterogeneity (14). Subgroup analysis was performed based on WHO classification of world regions (African/American/Eastern Mediterranean/European/ South-East Asian/ Western Pacific), type of population (college students/ general adult/ healthcare/ high risk/ parents and caregivers), gender (male/ female), vaccines effectiveness (at 90% effective/ at 95% effective) and survey time (first survey/ second survey). The high-risk population represented people most at risk of exposure, such as teachers and school students, detained people, patients, and pregnant and breastfeeding women.
Reporting Bias Assessment
The risk of bias was assessed by nine criteria (15): (1) appropriateness of sample frame (2) appropriateness of study participants sampled (3) adequate of sample size (4) description of study subjects and the setting (5) sample size justification, power description, or variance and effect estimates (6) valid methods for the identification of the condition (7) a standard and reliable condition measured (8) appropriateness of statistical analysis (9) adequate of response rate.
The criteria of the risk assessment were represented by “yes,” “no,” “unclear” or “not available.” The score for yes was one (1) and zero (0) for the rest. The risk of bias was considered low when the total score was more than 70%, moderate when 50–69%, and high when up to 0–49% (16).
Results
Study Selection
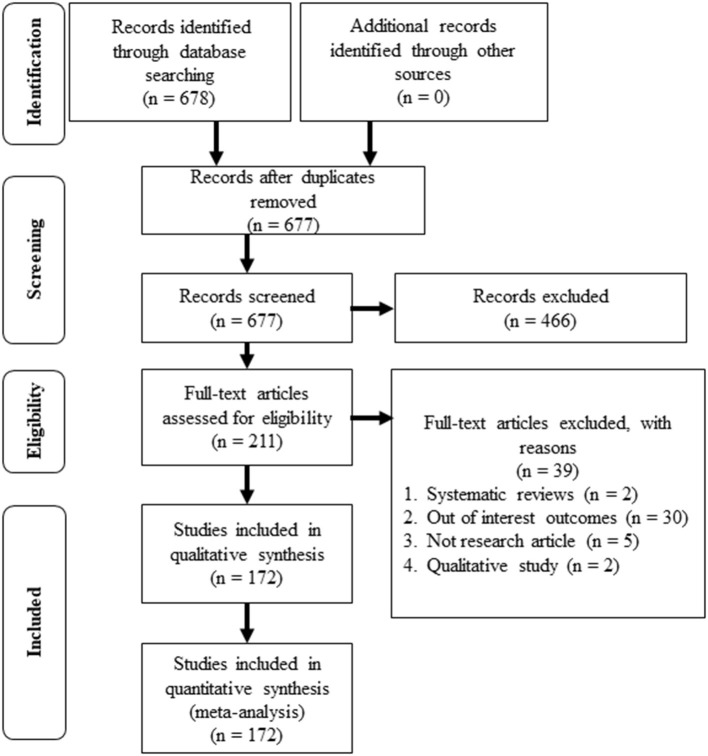
The primary search through the database had identified 678 studies. One duplicated study was removed, and 677 studies were screened for the titles and the abstracts. A total of 466 studies were excluded, and 211 full-text articles were assessed for eligibility. Thirty-nine studies were excluded for systematic review articles, out of interest outcomes, not research articles, and qualitative study articles. As a result, a total of 172 studies that met the inclusion and exclusion criteria were included for the review and meta-analysis (Figure 1).
Figure 1.
PRISMA flowchart of the review.
Study Characteristics
The articles of included studies were published in 2020 (n = 18) and in 2021 (n = 154). The studies involved 814,691 participants from 50 countries across six regions in the world represented as African region (n = 14) (17–30), American region (n = 34) (7, 31–63), Eastern Mediterranean region (n = 33) (8, 64–95), European region (n = 46) (96–141), South-East Asian region (n = 8) (5, 142–148) and Western Pacific region (n = 31) (149–179). There were two global studies (180, 181), one study combined countries in the European region and Western Pacific region (182), one study involved countries in the American region and Western Pacific region (183), one study involved the African region and Middle East countries (184) and one study involved countries in the African region, American region and South-East Asian region (185).
Most of the studies were designed as cross-sectional studies (n = 140), cohort studies (n = 4), descriptive studies (n = 23), longitudinal studies (n = 4) and combination of cross-sectional and longitudinal study (n = 1). A total of 28 studies applied probability sampling (random sampling, systematic sampling, stratified sampling, clustered sampling, and multistage sampling), 54 studies applied non-probability sampling (convenience sampling, quota sampling, purposive sampling, and snowball sampling), and 90 studies did not provide the sampling method (Supplementary Table 1).
Subgroup analysis involved the six regions in the world (African, American, Eastern Mediterranean, European, South-East Asian, and Western Pacific), population (college students, general adult population, healthcare workers, high-risk population, and parents and caregivers), gender, vaccines effectiveness (at 90 and 95%), and survey time.
Risk of Bias in Studies
The J.B.I. quality assessment showed that 60 studies were at low risk of bias, 47 studies were at moderate bias, and 65 studies were at high risk of bias (Supplementary Table 2). All the studies were included. The pooled proportion of COVID-19 vaccines acceptance for the studies with low, moderate, and high risk of bias were 0.59 (95% CI: 0.54, 0.65), 0.61 (95% CI: 0.57, 0.66) and 0.63 (95% CI: 0.58, 0.68), respectively.
Results of Total and Subgroup Studies
In Table 1, the total estimated proportion by COVID-19 vaccines acceptance pooled from 170 studies was 0.61 (95% CI: 0.59,0.64). The estimated pooled proportion by study designs of cohort study, cross sectional study, descriptive study, longitudinal study and combination study of longitudinal and cross-sectional were 0.77 (95% CI: 0.73, 0.81), 0.60 (95% CI: 0.57, 0.63), 0.69 (95% CI: 0.62, 0.75), 0.64 (95% CI: 0.49, 0.78) and 0.39 (95% CI: 0.37, 0.40) respectively. Meanwhile, the pooled proportion for probability sampling studies was 0.61 (95% CI: 0.55, 0.67) and 0.62 (95% CI: 0.57, 0.66) for non-probability sampling studies.
Table 1.
The outcome measures of COVID-19 vaccines acceptance.
| Outcome | No. of studies | Proportion (95% CI) | I2 (%) | p-valuea | I2 diff (%) | p-valuea | |
|---|---|---|---|---|---|---|---|
| Vaccines acceptance | 170 | 0.61 (0.59, 0.64) |
100 | p < 0.001 | |||
| Subgroup: | |||||||
| Study designs | Total | 170 | 0.61 (0.59, 0.64) |
100 | p < 0.001 | 99.1 | p < 0.001 |
| Cohort | 4 | 0.77 (0.73, 0.81) |
90 | p < 0.001 | |||
| Cross sectional | 138 | 0.60 (0.57, 0.63) |
100 | p < 0.001 | |||
| Descriptive | 23 | 0.69 (0.62, 0.75) |
100 | p < 0.001 | |||
| Longitudinal | 4 | 0.64 (0.49, 0.78) |
99 | p < 0.001 | |||
| Combination | 1 | 0.39 (0.37, 0.40) |
NA | NA | |||
| Sampling methods | Total | 81 | 0.61 (0.58, 0.65) |
100 | p < 0.001 | 0 | 0.82 |
| Probability | 27 | 0.61 (0.55, 0.67) |
100 | p < 0.001 | |||
| Non-probability | 54 | 0.62 (0.57, 0.66) |
100 | p < 0.001 | |||
| Regions | Total | 165 | 0.62 (0.59, 0.64) |
100 | p < 0.001 | 71.5 | 0.004 |
| African | 15 | 0.53 (0.39, 0.67) |
100 | p < 0.001 | |||
| American | 35 | 0.62 (0.57, 0.67) |
100 | p < 0.001 | |||
| Eastern Mediterranean | 33 | 0.52 (0.45, 0.59) |
100 | p < 0.001 | |||
| European | 46 | 0.65 (0.59, 0.71) |
100 | p < 0.001 | |||
| South-East Asian | 9 | 0.74 (0.64, 0.84) |
100 | p < 0.001 | |||
| Western Pacific | 32 | 0.66 (0.60, 0.73) |
100 | p < 0.001 | |||
| Population | Total | 170 | 0.61 (0.59, 0.64) |
100 | p < 0.001 | 0 | 0.66 |
| College students | 15 | 0.62 (0.52, 0.73) |
100 | p < 0.001 | |||
| General adult | 105 | 0.61 (0.58, 0.65) |
100 | p < 0.001 | |||
| Healthcare workers | 33 | 0.63 (0.56, 0.71) |
100 | p < 0.001 | |||
| High risks | 23 | 0.61 (0.52, 0.70) |
100 | p < 0.001 | |||
| Parents and caregivers | 7 | 0.52 (0.40, 0.65) |
99 | p < 0.001 | |||
| Gender | Total | 89 | 0.60 (0.56, 0.65) |
100 | p < 0.001 | 44.8 | 0.18 |
| Male | 89 | 0.64 (0.57, 0.71) |
100 | p < 0.001 | |||
| Female | 89 | 0.57 (0.48, 0.65) |
100 | p < 0.001 | |||
| Vaccines effectiveness | Total | 6 | 0.71 (0.63, 0.79) |
100 | p < 0.001 | 36.5 | 0.21 |
| At 90% effective | 3 | 0.62 (0.40, 0.84) |
100 | p < 0.001 | |||
| At 95% effective | 5 | 0.77 (0.69, 0.85) |
100 | p < 0.001 | |||
| Survey time | Total | 7 | 0.65 (0.54, 0.75) |
100 | p < 0.001 | 0 | 0.64 |
| First survey | 7 | 0.68 (0.56, 0.79) |
99 | p < 0.001 | |||
| Second survey | 7 | 0.62 (0.43, 0.81) |
100 | p < 0.001 | |||
Test for overall effect.
By subgroup analyses, the total proportion by regions was [0.62 (95% CI: 0.59, 0.64)] pooled from 165 studies, and the region with the highest proportion of COVID-19 vaccines acceptance was in South-East Asia [0.74 (95% CI: 0.64, 0.84)] which pooled from nine studies and the lowest vaccines acceptance was in Eastern Mediterranean [0.52 (95% CI: 0.45, 0.59)]. The total proportion by population was pooled from 170 studies [0.61 (95% CI: 0.59, 0.64)]. Population comparison showed that the proportion of COVID-19 vaccines acceptance was similar, which range 0.61 to 0.63 among college students, general adults, healthcare workers, and high-risk populations. However, the population of parents and caregivers was the lowest in the proportion of COVID-19 vaccines acceptance [0.52 (95% CI: 0.40, 0.65)].
The pooled proportion of COVID-19 vaccines acceptance by gender from 89 studies was [0.60 (95% CI: 0.56, 0.65)] and was higher in male [0.64 (95% CI: 0.57, 0.71)] compared to female [0.57 (95% CI: 0.48, 0.65)]. The 95% vaccines effectiveness showed higher in proportion of COVID-19 vaccines acceptance [0.77 (95% CI: 0.69, 0.85)] compared to 90% of vaccines effective [0.62 (95% CI: 0.40, 0.84)] with the total proportion by vaccines effectiveness was [0.71 (95% CI: 0.63, 0.79)] from six studies. The total pooled proportion from seven studies by survey time was 0.65 (95% CI: 0.54, 0.75). The proportion of COVID-19 vaccines acceptance was reduced in the second survey [0.62 (95% CI:0.43, 0.81)] compared to the first survey [0.68 (95% CI: 0.56, 0.79)]. In China and Australia, the surveys were repeated based on two epidemic phases, namely severe epidemic phase and well-contained phase (149, 171, 172). A study in Saudi Arabia performed the survey before and after the interim report of the efficacy rate of the RNA BNT162b2 vaccine (94).
All data for each outcome measure had considerable heterogeneity by the random-effects model (I2 > 99%). The heterogeneity of subgroups differences showed that the acceptance of the vaccine was with considerable heterogeneity; substantial heterogeneity for regions, moderate heterogeneity for gender and vaccines effectiveness. Heterogeneity might not be important for population and survey time (Table 1). The results were also presented in forest plots (Supplementary Figures 1–8).
Discussion
Vaccination is an essential approach for tackling the COVID-19 pandemic by achieving herd immunity in the population. The effectiveness of this approach depends on vaccination acceptance in the population. In the current review, the pooled proportion of COVID-19 vaccine acceptance from 170 studies worldwide involving 50 countries was 61% (95% CI: 59, 64%). This finding was lower compared to a previous estimate of COVID-19 vaccine acceptance [73.31% (95% CI: 70.52%, 76.01%)], which involved 38 studies across 36 countries with limited data from low-income countries (186). Concerns about the vaccine's safety, efficacy, and side effects, trust in the government or related authorities (186), and religious beliefs (187) were primary factors that influenced vaccine acceptance. The subgroup analyses of this current review also determined variability in vaccine acceptance, which ranged from 52 to 77%.
The pooled proportion of COVID-19 vaccine acceptance among regions ranged from 52 to 74%, with Southeast Asia the highest and the Eastern Mediterranean the lowest. This result was supported by a review that reported vaccine acceptance of over 90% in Southeast Asia and the lowest proportions of acceptance in Middle East countries, with < 30% in Kuwait and Jordan (187). The low vaccine acceptance in the Middle East was related to widespread beliefs in conspiracies that negatively affected vaccination (188). Nevertheless, this current review also saw low vaccine acceptance in the African region. A study in Nigeria revealed that besides geographical location, which was associated with low vaccine acceptance, the other plausible reasons for this situation were low education levels, which led to poor health literacy, distrust in vaccines and the government, and cultural and religious beliefs, among others (18).
Vaccination acceptance was also higher in males than in females, which is in line with other reviews (187, 189). It was reported that males were less likely to believe conspiracy theories and more likely to perceive COVID-19 as dangerous (127). Females were found to express more concerns about the safety of vaccines and distrust in the quality and impartiality of vaccine information provided by healthcare professionals (190). However, a study with a higher proportion of females who accepted the COVID-19 vaccine reported that they perceived that vaccination was for the safety of families and communities. Widespread vaccination coverage could allow them to return to the previous work of routines and childcare arrangements (191).
The pooled proportion of COVID-19 vaccine acceptance for the population groups varied from 52 to 63%. Unsurprisingly, healthcare workers showed the highest proportion of vaccine acceptance. Since healthcare workers were among the first to receive COVID-19 vaccines, their attitude or perception toward COVID-19 vaccines would affect the other population's decisions to recommend the vaccination to friends, families, and their patients (192). Similar proportions (61 to 62%) were also seen in college students, the general adult public, and high-risk populations. As reported by other studies, challenges threatening vaccination uptake in a population include media misinformation, especially from social media (189), and widely broadcast rumors, myths, and inaccurate beliefs regarding vaccines by the anti-vaccine community (193). Confusing information may affect people's awareness of vaccination, especially those who lack sufficient knowledge concerning COVID-19 vaccines (194). For parents and caregivers, the proportion who accepted vaccination for their children was low (52%), and this might be influenced by insufficient clinical data on vaccine safety and efficacy on children (181) and their concern that young children are likelier to suffer side effects (195). Many COVID-19 vaccines are still not approved for children younger than 12, so parents may think vaccines are unsafe for young children.
The decision to accept COVID-19 vaccination was also influenced by vaccine effectiveness (196); this review showed that people were willing to take vaccines with higher efficacy. The interim result of a living systematic review showed that the effectiveness of the COVID-19 vaccine after one dose varied between 16.9 and 91.2%. While the effectiveness increased to between 61.7 and 98.6% after completing the second dose (197). However, insufficient evidence for COVID-19 vaccine effectiveness has been reported as a leading reason for reduced confidence in vaccines among the general population (37, 185).
The time during which the survey was conducted showed that the acceptance of the COVID-19 vaccine changed over time. All the studies showed evidence that the COVID-19 vaccine acceptance changed over time. The proportion of vaccine acceptance was reduced in the second survey. A global review of vaccine acceptance also showed a similar pattern. In a review, countries such as France, Italy, and China established a decreased proportion of vaccine acceptance in their second and third surveys.
Conversely, the United States showed an increased pattern of vaccine acceptance in the second and third surveys. The situation was different in the United Kingdom, which showed that the proportion was high in the first survey, increased further in the second survey, and then decreased for the third and fourth surveys before increasing again in the fifth survey. Still, in the fifth survey, the proportion of vaccine acceptance was not as high as in the first survey (187). It has also been reported that reduced vaccine acceptance is related to increased serious side effects of the vaccines (196).
Media and public service messaging, particularly fear appraisal-framed public service messages compared to safety benefits public service messages, influence willingness to obtain the COVID-19 vaccine. However, conspiracy theories circulated in the media by vaccination-averse people about vaccine side effects impact people's decision to get vaccinated (198). The hesitation in vaccination may be due to overestimating perceived risk in the COVID-19 pandemic, messaging fatigue, and desensitization caused by repeated exposure to information. Consequently, overloading information especially social media confuses people and impairs their ability to differentiate between reliable sources and incorrect information (189).
Limitations of the Review
The search was limited to articles published in English only due to limited time, human, and financial resources to translate works published in other languages; this may have limited this review's generalizability. To have a more comprehensive assessment of the data, the authors decided to include all the available studies regardless of whether the quality of the data was low, moderate, or high, based on the assessment of the risk of bias. Furthermore, most of the research studies included in this review were cross-sectional studies, which can be thought of as visuals of vaccine hesitancy status in each country/region. They have different sampling strategies, which may explain some of the differences in vaccine acceptance rates reported in different studies from the same country. As a result, the findings should be regarded with caution, as they are unable to forecast future changes in vaccine acceptance rates. The other limitation was the sole dependence on the MEDLINE (PubMed) database in the search study. MEDLINE was reported as the best single source for retrieval of a systematic review, with an 89.7% inclusion rate that provided free-of-charge and open-access articles (199). However, it is also recommended to search extensively for studies using several databases to reduce possible biases in the included studies.
Conclusion
The rate of COVID-19 vaccine acceptance varied by region, population type, gender, vaccine effectiveness, and survey time, with an overall pooled proportion of 61%. A high level of acceptance of vaccination is required to achieve herd immunity for the disease. Many vaccination campaigns and programs are available globally to enhance public awareness to access and accept the COVID-19 vaccine to reach herd immunity and control the pandemic.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
RCY and MNN: conceptualization, methodology, and validation. RCY: software, formal analysis, data curation, and writing—original draft preparation. MNN and RCY: writing—review and editing. MNN and YMA: visualization and supervision. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.783982/full#supplementary-material
Forest plots of vaccines acceptance.
Forest plots of vaccines acceptance by study designs.
Forest plots of vaccines acceptance by sampling methods.
Forest plots of vaccines acceptance by regions.
Forest plots of vaccines acceptance by population.
Forest plots of vaccines acceptance by gender.
Forest plots of vaccines acceptance by vaccines effectiveness.
Forest plots of vaccines acceptance by survey time.
Characteristics of the included studies.
J.B.I Critical appraisal checklist for studies reporting prevalence data.
References
- 1.WHO . Weekly Epidemiological Update On COVID-19-31 August 2021. Edition 55 ed. World Health Organization; (2021). [Google Scholar]
- 2.WHO . Tracking SARS-CoV-2 Variants. World Health Organization; (2021). [Google Scholar]
- 3.WHO . Status of COVID-19 Vaccines Within WHO EUL/PQ Evaluation Process. World Health Organization; (2021). [Google Scholar]
- 4.Mayo . Herd Immunity and COVID-19 (coronavirus): What You Need To Know. Mayo Foundation for Medical Education and Research (MFMER). (2021). Available online at: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/herd-immunity-and-coronavirus/art-20486808 (accessed July 14, 2021).
- 5.Syed Alwi SAR, Rafidah E, Zurraini A, Juslina O, Brohi IB, Lukas S. A survey on COVID-19 vaccine acceptance and concern among Malaysians. BMC Public Health. (2021) 21:1129. 10.1186/s12889-021-11071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. 10.1038/s41591-020-1124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. (2020) 26:100495. 10.1016/j.eclinm.2020.100495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mohaithef M, Padhi BK. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: A web-based national survey. J Multidiscip Healthc. (2020) 13:1657–63. 10.2147/JMDH.S276771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou Z, Tong Y, Du F, Lu L, Zhao S, Yu K, et al. Assessing COVID-19 vaccine hesitancy, confidence, and public engagement: a global social listening study. J Med Internet Res. (2021) 23:e27632. 10.2196/27632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qunaibi EA, Helmy M, Basheti I, Sultan I. A high rate of COVID-19 vaccine hesitancy in a large-scale survey on Arabs. Elife. (2021) 10:e68038. 10.7554/eLife.68038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aromataris E, Munn Z. JBI Manual For Evidence Synthesis. (2020). Available online at: https://synthesismanual.jbi.global. 10.46658/JBIMES-20-01 [DOI]
- 13.Review Manager (RevMan). 5.4.1. The Cochrane Collaboration (2020). [Google Scholar]
- 14.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook For Systematic Reviews Of Interventions Version 6.2. Cochrane. (2021). Available online at: www.training.cochrane.org/handbook (accessed February 2021).
- 15.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. (2015) 13:147–53. 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 16.Cristina da Silva Rosa B, Hernandez Alves Ribeiro César CP, Paranhos LR, Guedes-Granzotti RB, Lewis DR. Speech-language disorders in children with congenital Zika virus syndrome: a systematic review. Int J Pediatr Otorhinolaryngol. (2020) 138:110309. 10.1016/j.ijporl.2020.110309 [DOI] [PubMed] [Google Scholar]
- 17.Abebe H, Shitu S, Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect Drug Resist. (2021) 14:2015–25. 10.2147/IDR.S312116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adebisi YA, Alaran AJ, Bolarinwa OA, Akande-Sholabi W, Lucero-Prisno DE. When it is available, will we take it? social media users' perception of hypothetical COVID-19 vaccine in Nigeria. Pan Afr Med J. (2021) 38:230. 10.11604/pamj.2021.38.230.27325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeniyi OV, Stead D, Singata-Madliki M, Batting J, Wright M, Jelliman E, et al. Acceptance of COVID-19 vaccine among the healthcare workers in the Eastern Cape, South Africa: a cross sectional study. Vaccines. (2021) 9:6. 10.3390/vaccines9060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bongomin F, Olum R, Andia-Biraro I, Nakwagala FN, Hassan KH, Nassozi DR, et al. COVID-19 vaccine acceptance among high-risk populations in Uganda. Ther Adv Infect Dis. (2021) 8:20499361211024376. 10.1177/20499361211024376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinga JN, Sinda LK, Titanji VPK. Assessment of vaccine hesitancy to a COVID-19 vaccine in cameroonian adults and its global implication. Vaccines. (2021) 9:2. 10.3390/vaccines9020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ditekemena JD, Nkamba DM, Mutwadi A, Mavoko HM, Siewe Fodjo JN, Luhata C, et al. COVID-19 vaccine acceptance in the democratic Republic of Congo: a cross-sectional survey. Vaccines. (2021) 9:2. 10.3390/vaccines9020153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Echoru I, Ajambo PD, Keirania E, Bukenya EEM. Sociodemographic factors associated with acceptance of COVID-19 vaccine and clinical trials in Uganda: a cross-sectional study in western Uganda. BMC Public Health. (2021) 21:1106. 10.1186/s12889-021-11197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handebo S, Wolde M, Shitu K, Kassie A. Determinant of intention to receive COVID-19 vaccine among school teachers in Gondar City, Northwest Ethiopia. PLoS ONE. (2021) 16:e0253499. 10.1371/journal.pone.0253499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabamba Nzaji M, Kabamba Ngombe L, Ngoie Mwamba G, Banza Ndala DB, Mbidi Miema J, Luhata Lungoyo C, et al. Acceptability of vaccination against COVID-19 among healthcare workers in the democratic Republic of the Congo. Pragmat Obs Res. (2020) 11:103–9. 10.2147/POR.S271096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanyike AM, Olum R, Kajjimu J, Ojilong D, Akech GM, Nassozi DR, et al. Acceptance of the coronavirus disease-2019 vaccine among medical students in Uganda. Trop Med Health. (2021) 49:37. 10.1186/s41182-021-00331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamptey E, Serwaa D, Appiah AB. A nationwide survey of the potential acceptance and determinants of COVID-19 vaccines in Ghana. Clin Exp Vaccine Res. (2021) 10:183–90. 10.7774/cevr.2021.10.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesele M. COVID-19 vaccination acceptance and its associated factors in Sodo Town, Wolaita Zone, Southern Ethiopia: cross-sectional study. Infect Drug Resist. (2021) 14:2361–7. 10.2147/IDR.S320771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mose A, Yeshaneh A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in Southwest Ethiopia: institutional-based cross-sectional study. Int J Gen Med. (2021) 14:2385–95. 10.2147/IJGM.S314346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zewude B, Habtegiorgis T. Willingness to take COVID-19 vaccine among people most at risk of exposure in Southern Ethiopia. Pragmat Obs Res. (2021) 12:37–47. 10.2147/POR.S313991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarado-Socarras JL, Vesga-Varela AL, Quintero-Lesmes DC, Fama-Pereira MM, Serrano-Diaz NC, Vasco M, et al. Perception of COVID-19 vaccination amongst physicians in Colombia. Vaccines. (2021) 9:3. 10.3390/vaccines9030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmody ER, Zander D, Klein EJ, Mulligan MJ, Caplan AL. Knowledge and attitudes toward COVID-19 and vaccines among a New York Haredi-Orthodox Jewish community. J Community Health. (2021) 46:1161–9. 10.1007/s10900-021-00995-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerda AA, García LY. Hesitation and refusal factors in individuals' decision-making processes regarding a Coronavirus Disease 2019 vaccination. Front Public Health. (2021) 9:626852. 10.3389/fpubh.2021.626852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubé È, Dionne M, Pelletier C, Hamel D, Gadio S. COVID-19 vaccination attitudes and intention among Quebecers during the first and second waves of the pandemic: findings from repeated cross-sectional surveys. Hum Vaccin Immunother. (2021) 17:3922–32. 10.1080/21645515.2021.1947096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehde DM, Roberts MK, Herring TE, Alschuler KN. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord. (2021) 49:102788. 10.1016/j.msard.2021.102788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann Intern Med. (2020) 173:964–73. 10.7326/M20-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatwood J, McKnight M, Fiscus M, Hohmeier KC, Chisholm-Burns M. Factors influencing likelihood of COVID-19 vaccination: a survey of Tennessee adults. Am J Health Syst Pharm. (2021) 78:879–89. 10.1093/ajhp/zxab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hetherington E, Edwards SA, MacDonald SE, Racine N, Madigan S, McDonald S, et al. SARS-CoV-2 vaccination intentions among mothers of children aged 9 to 12 years: a survey of the all our families cohort. CMAJ Open. (2021) 9:E548–55. 10.9778/cmajo.20200302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaramillo-Monge J, Obimpeh M, Vega B, Acurio D, Boven A, Verhoeven V, et al. COVID-19 vaccine acceptance in Azuay Province, Ecuador: a cross-sectional online survey. Vaccines. (2021) 9:6. 10.3390/vaccines9060678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson KD, Akingbola O, Anderson J, Hart J, Chapple A, Woods C, et al. Combatting a “Twin-demic”: A quantitative assessment of COVID-19 and influenza vaccine hesitancy in primary care patients. Health Promot Perspect. (2021) 11:179–85. 10.34172/hpp.2021.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelkar AH, Blake JA, Cherabuddi K, Cornett H, McKee BL, Cogle CR. Vaccine enthusiasm and hesitancy in cancer patients and the impact of a webinars. Healthcare. (2021) 9:3. 10.3390/healthcare9030351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuter BJ, Browne S, Momplaisir FM, Feemster KA, Shen AK, Green-McKenzie J, et al. Perspectives on the receipt of a COVID-19 vaccine: a survey of employees in two large hospitals in Philadelphia. Vaccine. (2021) 39:1693–700. 10.1016/j.vaccine.2021.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucia VC, Kelekar A, Afonso NM. COVID-19 vaccine hesitancy among medical students. J Public Health. (2020) 43:445-9. 10.1093/pubmed/fdaa230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascarenhas AK, Lucia VC, Kelekar A, Afonso NM. Dental students' attitudes and hesitancy toward COVID-19 vaccine. J Dent Educ. (2021). 10.1002/jdd.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikolovski J, Koldijk M, Weverling GJ, Spertus J, Turakhia M, Saxon L, et al. Factors indicating intention to vaccinate with a COVID-19 vaccine among older U.S. adults. PLoS ONE. (2021) 16:e0251963. 10.1371/journal.pone.0251963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olanipekun T, Abe T, Effoe V, Kagbo-Kue S, Chineke I, Ivonye C, et al. Attitudes and perceptions towards Coronavirus Disease 2019 (COVID-19) vaccine acceptance among recovered African American patients. J Gen Intern Med. (2021) 36:2186–8. 10.1007/s11606-021-06787-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parente DJ, Ojo A, Gurley T, LeMaster JW, Meyer M, Wild DM, et al. Acceptance of COVID-19 vaccination among health system personnel. J Am Board Fam Med. (2021) 34:498–508. 10.3122/jabfm.2021.03.200541 [DOI] [PubMed] [Google Scholar]
- 48.Piltch-Loeb R, Savoia E, Goldberg B, Hughes B, Verhey T, Kayyem J, et al. Examining the effect of information channel on COVID-19 vaccine acceptance. PLoS ONE. (2021) 16:e0251095. 10.1371/journal.pone.0251095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pogue K, Jensen JL, Stancil CK, Ferguson DG, Hughes SJ, Mello EJ, et al. Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines. (2020) 8:4. 10.3390/vaccines8040582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Racey CS, Donken R, Porter I, Albert A, Bettinger JA, Mark J, et al. Intentions of public school teachers in British Columbia, Canada to receive a COVID-19 vaccine. Vaccine: X. (2021) 8:100106. 10.1016/j.jvacx.2021.100106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. (2020) 38:6500–7. 10.1016/j.vaccine.2020.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salmon DA, Dudley MZ, Brewer J, Kan L, Gerber JE, Budigan H, et al. COVID-19 vaccination attitudes, values and intentions among United States adults prior to emergency use authorization. Vaccine. (2021) 39:2698–711. 10.1016/j.vaccine.2021.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma M, Davis RE, Wilkerson AH. COVID-19 vaccine acceptance among college students: a theory-based analysis. Int J Environ Res Public Health. (2021) 18:9. 10.3390/ijerph18094617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw J, Stewart T, Anderson KB, Hanley S, Thomas SJ, Salmon DA, et al. Assessment of U.S. health care personnel (HCP) attitudes towards COVID-19 vaccination in a large university health care system. Clin Infect Dis. (2021) 73:1776–83. 10.1093/cid/ciab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, et al. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. (2021) 9:2. 10.3390/vaccines9020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shih SF, Wagner AL, Masters NB, Prosser LA, Lu Y, Zikmund-Fisher BJ. Vaccine hesitancy and rejection of a vaccine for the novel Coronavirus in the United States. Front Immunol. (2021) 12:558270. 10.3389/fimmu.2021.558270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stern MF, Piasecki AM, Strick LB, Rajeshwar P, Tyagi E, Dolovich S, et al. Willingness to receive a COVID-19 vaccination among incarcerated or detained persons in correctional and detention facilities - four states, September-December 2020. MMWR Morb Mortal Wkly Rep. (2021) 70:473–7. 10.15585/mmwr.mm7013a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsapepas D, Husain SA, King KL, Burgos Y, Cohen DJ, Mohan S. Perspectives on COVID-19 vaccination among kidney and pancreas transplant recipients living in New York City. Am J Health Syst Pharm. (2021) 78:2040–5. 10.1093/ajhp/zxab272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urrunaga-Pastor D, Bendezu-Quispe G, Herrera-Añazco P, Uyen-Cateriano A, Toro-Huamanchumo CJ, Rodriguez-Morales AJ, et al. Cross-sectional analysis of COVID-19 vaccine intention, perceptions and hesitancy across Latin America and the Caribbean. Travel Med Infect Dis. (2021) 41:102059. 10.1016/j.tmaid.2021.102059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viswanath K, Bekalu M, Dhawan D, Pinnamaneni R, Lang J, McLoud R. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health. (2021) 21:818. 10.1186/s12889-021-10862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang XM, Hollen C, Yang Q, Brumbach BH, Spain RI, Wooliscroft L. COVID-19 vaccination willingness among people with multiple sclerosis. Mult Scler J Exp Transl Clin. (2021) 7:20552173211017159. 10.1177/20552173211017159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Dobalian A, Ward KD. COVID-19 vaccine hesitancy and its determinants among adults with a history of tobacco or marijuana use. J Community Health. (2021) 46:1090–8. 10.1007/s10900-021-00993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J Community Health. (2021) 46:270–7. 10.1007/s10900-020-00958-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abu-Farha R, Mukattash T, Itani R, Karout S, Khojah H, Al-Mahmood A, et al. Willingness of Middle Eastern public to receive COVID-19 vaccines. Saudi Pharm J. (2021) 29:734-9. 10.1016/j.jsps.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmed MAM, Colebunders R, Gele AA, Farah AA, Osman S, Guled IA, et al. COVID-19 vaccine acceptability and adherence to preventive measures in Somalia: results of an online survey. Vaccines. (2021) 9:6. 10.3390/vaccines9060543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alabdulla M, Reagu SM, Al-Khal A, Elzain M, Jones RM. COVID-19 vaccine hesitancy and attitudes in Qatar: a national cross-sectional survey of a migrant-majority population. Influenza Other Respir Viruses. (2021) 15:361–70. 10.1111/irv.12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.AlAwadhi E, Zein D, Mallallah F, Bin Haider N, Hossain A. Monitoring COVID-19 vaccine acceptance in Kuwait during the pandemic: results from a national serial study. Risk Manag Healthc Policy. (2021) 14:1413–29. 10.2147/RMHP.S300602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alfageeh EI, Alshareef N, Angawi K, Alhazmi F, Chirwa GC. Acceptability of a COVID-19 vaccine among the Saudi population. Vaccines. (2021) 9:3. 10.3390/vaccines9030226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Marshoudi S, Al-Balushi H, Al-Wahaibi A, Al-Khalili S, Al-Maani A, Al-Farsi N, et al. Knowledge, attitudes, and practices (KAP) toward the COVID-19 vaccine in Oman: a pre-campaign cross-sectional study. Vaccines. (2021) 9:6. 10.3390/vaccines9060602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Metwali BZ, Al-Jumaili AA, Al-Alag ZA, Sorofman B. Exploring the acceptance of COVID-19 vaccine among healthcare workers and general population using health belief model. J Eval Clin Pract. (2021) 27:1112–22. 10.1111/jep.13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Mistarehi AH, Kheirallah KA, Yassin A, Alomari S, Aledrisi MK, Bani Ata EM, et al. Determinants of the willingness of the general population to get vaccinated against COVID-19 in a developing country. Clin Exp Vaccine Res. (2021) 10:171–82. 10.7774/cevr.2021.10.2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Mulla R, Abu-Madi M, Talafha QM, Tayyem RF, Abdallah AM. COVID-19 vaccine hesitancy in a representative education sector population in Qatar. Vaccines. (2021) 9:6. 10.3390/vaccines9060665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alobaidi S. Predictors of intent to receive the COVID-19 vaccination among the population in the Kingdom of Saudi Arabia: a survey study. J Multidiscip Healthc. (2021) 14:1119–28. 10.2147/JMDH.S306654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Qerem WA, Jarab AS. COVID-19 vaccination acceptance and its associated factors among a Middle Eastern population. Front Public Health. (2021) 9:632914. 10.3389/fpubh.2021.632914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alqudeimat Y, Alenezi D, AlHajri B, Alfouzan H, Almokhaizeem Z, Altamimi S, et al. Acceptance of a COVID-19 vaccine and its related determinants among the general adult population in Kuwait. Med Princ Pract. (2021) 30:262–71. 10.1159/000514636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Sanafi M, Sallam M. Psychological determinants of COVID-19 vaccine acceptance among healthcare workers in Kuwait: a cross-sectional study using the 5C and vaccine conspiracy beliefs scales. Vaccines. (2021) 9:7. 10.3390/vaccines9070701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alshahrani SM, Dehom S, Almutairi D, Alnasser BS, Alsaif B, Alabdrabalnabi AA, et al. Acceptability of COVID-19 vaccination in Saudi Arabia: a cross-sectional study using a web-based survey. Hum Vaccin Immunother. (2021) 17:3338–47. 10.1080/21645515.2021.1936869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baghdadi LR, Alghaihb SG, Abuhaimed AA, Alkelabi DM, Alqahtani RS. Healthcare workers' perspectives on the upcoming COVID-19 vaccine in terms of their exposure to the influenza vaccine in Riyadh, Saudi Arabia: a cross-sectional study. Vaccines. (2021) 9:5. 10.3390/vaccines9050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaudhary FA, Ahmad B, Khalid MD, Fazal A, Javaid MM, Butt DQ. Factors influencing COVID-19 vaccine hesitancy and acceptance among the Pakistani population. Hum Vaccin Immunother. (2021) 17:3365–70. 10.1080/21645515.2021.1944743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dror AA, Daoud A, Morozov NG, Layous E, Eisenbach N, Mizrachi M, et al. Vaccine hesitancy due to vaccine country of origin, vaccine technology, and certification. Eur J Epidemiol. (2021) 36:709–14. 10.1007/s10654-021-00758-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dror AA, Eisenbach N, Taiber S, Morozov NG, Mizrachi M, Zigron A, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. (2020) 35:775–9. 10.1007/s10654-020-00671-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ. Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. PLoS ONE. (2021) 16:e0250555. 10.1371/journal.pone.0250555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elhadi M, Alsoufi A, Alhadi A, Hmeida A, Alshareea E, Dokali M, et al. Knowledge, attitude, and acceptance of healthcare workers and the public regarding the COVID-19 vaccine: a cross-sectional study. BMC Public Health. (2021) 21:955. 10.1186/s12889-021-10987-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fares S, Elmnyer MM, Mohamed SS, Elsayed R. COVID-19 vaccination perception and attitude among healthcare workers in Egypt. J Prim Care Community Health. (2021) 12:21501327211013303. 10.1177/21501327211013303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasrine Al Halabi C, Obeid S, Sacre H, Akel M, Hallit R, Salameh P, et al. Attitudes of Lebanese adults regarding COVID-19 vaccination. BMC Public Health. (2021) 21:998. 10.1186/s12889-021-10902-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maraqa B, Nazzal Z, Rabi R, Sarhan N, Al-Shakhra K, Al-Kaila M. COVID-19 vaccine hesitancy among health care workers in Palestine: a call for action. Prev Med. (2021) 149:106618. 10.1016/j.ypmed.2021.106618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohamad O, Zamlout A, AlKhoury N, Mazloum AA, Alsalkini M, Shaaban R. Factors associated with the intention of Syrian adult population to accept COVID19 vaccination: a cross-sectional study. BMC Public Health. (2021) 21:1310. 10.1186/s12889-021-11361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qattan AMN, Alshareef N, Alsharqi O, Al Rahahleh N, Chirwa GC, Al-Hanawi MK. Acceptability of a COVID-19 vaccine among healthcare workers in the Kingdom of Saudi Arabia. Front Med. (2021) 8:644300. 10.3389/fmed.2021.644300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rabi R, Maraqa B, Nazzal Z, Zink T. Factors affecting nurses' intention to accept the COVID-19 vaccine: a cross-sectional study. Public Health Nurs. (2021). 10.1111/phn.12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saied SM, Saied EM, Kabbash IA, Abdo SAE. Vaccine hesitancy: beliefs and barriers associated with COVID-19 vaccination among Egyptian medical students. J Med Virol. (2021) 93:4280–91. 10.1002/jmv.26910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sallam M, Dababseh D, Eid H, Al-Mahzoum K, Al-Haidar A, Taim D, et al. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other Arab countries. Vaccines. (2021) 9:1. 10.3390/vaccines9010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sallam M, Dababseh D, Eid H, Hasan H, Taim D, Al-Mahzoum K, et al. Low COVID-19 vaccine acceptance is correlated with conspiracy beliefs among university students in Jordan. Int J Environ Res Public Health. (2021) 18:5. 10.3390/ijerph18052407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shmueli L. Predicting intention to receive COVID-19 vaccine among the general population using the health belief model and the theory of planned behavior model. BMC Public Health. (2021) 21:804. 10.1186/s12889-021-10816-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Temsah MH, Barry M, Aljamaan F, Alhuzaimi A, Al-Eyadhy A, Saddik B, et al. Adenovirus and RNA-based COVID-19 vaccines' perceptions and acceptance among healthcare workers in Saudi Arabia: a national survey. BMJ Open. (2021) 11:e048586. 10.1136/bmjopen-2020-048586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khaled SM, Petcu C, Bader L, Amro I, Al-Hamadi A, Al Assi M, et al. Prevalence and potential determinants of COVID-19 vaccine hesitancy and resistance in Qatar: results from a nationally representative survey of Qatari nationals and migrants between December 2020 and January 2021. Vaccines. (2021) 9:5. 10.3390/vaccines9050471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abuown A, Ellis T, Miller J, Davidson R, Kachwala Q, Medeiros M, et al. COVID-19 vaccination intent among London healthcare workers. Occup Med. (2021) 71:211-4. 10.1093/occmed/kqab057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Babicki M, Mastalerz-Migas A. Attitudes toward vaccination against COVID-19 in Poland. a longitudinal study performed before and two months after the commencement of the population vaccination programme in Poland. Vaccines. (2021) 9:5. 10.3390/vaccines9050503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bendau A, Plag J, Petzold MB, Ströhle A. COVID-19 vaccine hesitancy and related fears and anxiety. Int Immunopharmacol. (2021) 97:107724. 10.1016/j.intimp.2021.107724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blanchard-Rohner G, Caprettini B, Rohner D, Voth HJ. Impact of COVID-19 and intensive care unit capacity on vaccination support: Evidence from a two-leg representative survey in the United Kingdom. J Virus Erad. (2021) 7:100044. 10.1016/j.jve.2021.100044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butter S, McGlinchey E, Berry E, Armour C. Psychological, social, and situational factors associated with COVID-19 vaccination intentions: a study of UK key workers and non-key workers. Br J Health Psychol. (2021) 27:13–29. 10.1111/bjhp.12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Del Riccio M, Boccalini S, Rigon L, Biamonte MA, Albora G, Giorgetti D, et al. Factors influencing SARS-CoV-2 vaccine acceptance and hesitancy in a population-based sample in Italy. Vaccines. (2021) 9:6. 10.3390/vaccines9060633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. (2020) 38:7002–6. 10.1016/j.vaccine.2020.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di Gennaro F, Murri R, Segala FV, Cerruti L, Abdulle A, Saracino A, et al. Attitudes towards Anti-SARS-CoV2 vaccination among healthcare workers: results from a national survey in Italy. Viruses. (2021) 13:3. 10.3390/v13030371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Di Giuseppe G, Pelullo CP, Della Polla G, Pavia M, Angelillo IF. Exploring the willingness to accept SARS-CoV-2 vaccine in a university population in Southern Italy, September to November 2020. Vaccines. (2021) 9:3. 10.3390/vaccines9030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fedele F, Aria M, Esposito V, Micillo M, Cecere G, Spano M, et al. COVID-19 vaccine hesitancy: a survey in a population highly compliant to common vaccinations. Hum Vaccin Immunother. (2021) 17:3348–54. 10.1080/21645515.2021.1928460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gagneux-Brunon A, Detoc M, Bruel S, Tardy B, Rozaire O, Frappe P, et al. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect. (2021) 108:168–73. 10.1016/j.jhin.2020.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gallè F, Sabella EA, Roma P, De Giglio O, Caggiano G, Tafuri S, et al. Knowledge and acceptance of COVID-19 vaccination among undergraduate students from Central and Southern Italy. Vaccines. (2021) 9:6. 10.3390/vaccines9060638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Graffigna G, Palamenghi L, Boccia S, Barello S. Relationship between citizens' health engagement and intention to take the COVID-19 vaccine in Italy: a mediation analysis. Vaccines. (2020) 8:4. 10.3390/vaccines8040576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grochowska M, Ratajczak A, Zdunek G, Adamiec A, Waszkiewicz P, Feleszko W, et al. comparison of the level of acceptance and hesitancy towards the influenza vaccine and the forthcoming COVID-19 vaccine in the medical community. Vaccines. (2021) 9:5. 10.3390/vaccines9050475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guaraldi F, Montalti M, Di Valerio Z, Mannucci E, Nreu B, Monami M, et al. Rate and predictors of hesitancy toward SARS-CoV-2 vaccine among type 2 diabetic patients: results from an Italian survey. Vaccines. (2021) 9:5. 10.3390/vaccines9050460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hammer CC, Cristea V, Dub T, Sivelä J. High but slightly declining COVID-19 vaccine acceptance and reasons for vaccine acceptance, Finland April to December 2020. Epidemiol Infect. (2021) 149:e123. 10.1017/S0950268821001114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holeva V, Parlapani E, Nikopoulou VA, Nouskas I, Diakogiannis I. COVID-19 vaccine hesitancy in a sample of Greek adults. Psychol Health Med. (2021) 27:113–9. 10.1080/13548506.2021.1948579 [DOI] [PubMed] [Google Scholar]
- 113.Ikiişik H, Akif Sezerol M, Taşçi Y, Maral I. COVID-19 vaccine hesitancy: a community-based research in Turkey. Int J Clin Pract. (2021) 75:e14336. 10.1111/ijcp.14336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janssen C, Maillard A, Bodelet C, Claudel AL, Gaillat J, Delory T, et al. Hesitancy towards COVID-19 vaccination among healthcare workers: a multi-centric survey in France. Vaccines. (2021) 9:6. 10.3390/vaccines9060547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaplan AK, Sahin MK, Parildar H, Adadan Guvenc I. The willingness to accept the COVID-19 vaccine and affecting factors among healthcare professionals: a cross-sectional study in Turkey. Int J Clin Pract. (2021) 75:e14226. 10.1111/ijcp.14226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Konopińska J, Obuchowska I, Lisowski Ł, Dub N, Kozera M, Rekas M. Intention to get COVID-19 vaccinations among ophthalmology residents in Poland: a cross-sectional survey. Vaccines. (2021) 9:4. 10.3390/vaccines9040371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kourlaba G, Kourkouni E, Maistreli S, Tsopela CG, Molocha NM, Triantafyllou C, et al. Willingness of Greek general population to get a COVID-19 vaccine. Glob Health Res Policy (2021) 6:3. 10.1186/s41256-021-00188-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.La Vecchia C, Negri E, Alicandro G, Scarpino V. Attitudes towards influenza vaccine and a potential COVID-19 vaccine in Italy and differences across occupational groups, September 2020. Med Lav. (2020) 111:445–8. 10.23749/mdl.v111i6.10813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ledda C, Costantino C, Cuccia M, Maltezou HC, Rapisarda V. Attitudes of healthcare personnel towards vaccinations before and during the COVID-19 pandemic. Int J Environ Res Public Health. (2021) 18:5. 10.3390/ijerph18052703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Malesza M, Wittmann E. Acceptance and intake of COVID-19 vaccines among older Germans. J Clin Med. (2021) 10:7. 10.3390/jcm10071388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murphy J, Vallières F, Bentall RP, Shevlin M, McBride O, Hartman TK, et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat Commun. (2021) 12:29. 10.1038/s41467-020-20226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nohl A, Afflerbach C, Lurz C, Brune B, Ohmann T, Weichert V, et al. Acceptance of COVID-19 vaccination among front-line health care workers: a nationwide survey of emergency medical services personnel from Germany. Vaccines. (2021) 9:5. 10.3390/vaccines9050424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Palamenghi L, Barello S, Boccia S, Graffigna G. Mistrust in biomedical research and vaccine hesitancy: the forefront challenge in the battle against COVID-19 in Italy. Eur J Epidemiol. (2020) 35:785–8. 10.1007/s10654-020-00675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pataka A, Kotoulas S, Stefanidou E, Grigoriou I, Tzinas A, Tsiouprou I, et al. Acceptability of healthcare professionals to get vaccinated against COVID-19 two weeks before initiation of national vaccination. Medicina. (2021) 57:6. 10.3390/medicina57060611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prati G. Intention to receive a vaccine against SARS-CoV-2 in Italy and its association with trust, worry and beliefs about the origin of the virus. Health Educ Res. (2020) 35:505–11. 10.1093/her/cyaa043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Puteikis K, Mameniškiene R. Factors associated with COVID-19 vaccine hesitancy among people with epilepsy in Lithuania. Int J Environ Res Public Health. (2021) 18:8. 10.3390/ijerph18084374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reno C, Maietti E, Fantini MP, Savoia E, Manzoli L, Montalti M, et al. Enhancing COVID-19 vaccines acceptance: results from a survey on vaccine hesitancy in Northern Italy. Vaccines. (2021) 9:4. 10.3390/vaccines9040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rodríguez-Blanco N, Montero-Navarro S, Botella-Rico JM, Felipe-Gómez AJ, Sánchez-Más J, Tuells J. Willingness to be vaccinated against COVID-19 in Spain before the start of vaccination: a cross-sectional study. Int J Environ Res Public Health. (2021) 18:10. 10.3390/ijerph18105272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. (2021) 6:e210–21. 10.1016/S2468-2667(21)00012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Serrazina F, Sobral Pinho A, Cabral G, Salavisa M, Correia AS. Willingness to be vaccinated against COVID-19: an exploratory online survey in a Portuguese cohort of multiple sclerosis patients. Mult Scler Relat Disord. (2021) 51:102880. 10.1016/j.msard.2021.102880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sherman SM, Smith LE, Sim J, Amlôt R, Cutts M, Dasch H, et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccin Immunother. (2021) 17:1612–21. 10.1080/21645515.2020.1846397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soares P, Rocha JV, Moniz M, Gama A, Laires PA, Pedro AR, et al. Factors associated with COVID-19 vaccine hesitancy. Vaccines. (2021) 9:3. 10.3390/vaccines9030300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stuckelberger S, Favre G, Ceulemans M, Nordeng H, Gerbier E, Lambelet V, et al. SARS-CoV-2 vaccine willingness among pregnant and breastfeeding women during the first pandemic wave: a Cross-sectional study in Switzerland. Viruses. (2021) 13:7. 10.3390/v13071199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Talarek E, Warzecha J, Banasiuk M, Banaszkiewicz A. Influenza vaccination coverage and intention to receive hypothetical Ebola and COVID-19 vaccines among medical students. Vaccines. (2021) 9:7. 10.3390/vaccines9070709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tavolacci MP, Dechelotte P, Ladner J. COVID-19 vaccine acceptance, hesitancy, and resistancy among university students in France. Vaccines. (2021) 9:6. 10.3390/vaccines9060654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trabucco Aurilio M, Mennini FS, Gazzillo S, Massini L, Bolcato M, Feola A, et al. Intention to Be Vaccinated for COVID-19 among Italian nurses during the Pandemic. Vaccines. (2021) 9:5. 10.3390/vaccines9050500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tran VD, Pak TV, Gribkova EI, Galkina GA, Loskutova EE, Dorofeeva VV, et al. Determinants of COVID-19 vaccine acceptance in a high infection-rate country: a cross-sectional study in Russia. Pharm Pract. (2021) 19:2276. 10.18549/PharmPract.2021.1.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vallée A, Fourn E, Majerholc C, Touche P, Zucman D. COVID-19 vaccine hesitancy among French people living with HIV. Vaccines. (2021) 9:4. 10.3390/vaccines9040302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Williams L, Flowers P, McLeod J, Young D, Rollins L, The catalyst project T . social patterning and stability of intention to accept a COVID-19 vaccine in Scotland: will those most at risk accept a vaccine? Vaccines. (2021) 9:1. 10.3390/vaccines9010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yilmaz M, Sahin MK. Parents' willingness and attitudes concerning the COVID-19 vaccine: a cross-sectional study. Int J Clin Pract. (2021) 75:e14364. 10.1111/ijcp.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yurttas B, Poyraz BC, Sut N, Ozdede A, Oztas M, Ugurlu S, et al. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol Int. (2021) 41:1105–14. 10.1007/s00296-021-04841-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akiful Haque MM, Rahman ML, Hossian M, Matin KF, Nabi MH, Saha S, et al. Acceptance of COVID-19 vaccine and its determinants: evidence from a large sample study in Bangladesh. Heliyon. (2021) 7:e07376. 10.1016/j.heliyon.2021.e07376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Front Public Health. (2020) 8:381. 10.3389/fpubh.2020.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jacob J, Stephen S, Issac A, Krishnan N, Vadakkethil Radhakrishnan R, Vijay VR, et al. Determinants of willingness for COVID-19 vaccine: Implications for enhancing the proportion of vaccination among Indians. Cureus. (2021) 13:e15271. 10.7759/cureus.15271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kumari A, Ranjan P, Chopra S, Kaur D, Kaur T, Upadhyay AD, et al. Knowledge, barriers and facilitators regarding COVID-19 vaccine and vaccination programme among the general population: a cross-sectional survey from one thousand two hundred and forty-nine participants. Diabetes Metab Syndr. (2021) 15:987–92. 10.1016/j.dsx.2021.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Panda DS, Giri RK, Nagarajappa AK, Basha S. Covid-19 vaccine, acceptance, and concern of safety from public perspective in the state of Odisha, India. Hum Vaccin Immunother. (2021) 17:3333–7. 10.1080/21645515.2021.1924017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wirawan GBS, Mahardani P, Cahyani MRK, Laksmi N, Januraga PP. Conspiracy beliefs and trust as determinants of COVID-19 vaccine acceptance in Bali, Indonesia: Cross-sectional study. Pers Individ Dif. (2021) 180:110995. 10.1016/j.paid.2021.110995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wong LP, Alias H, Wong PF, Lee HY, AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum Vaccin Immunother. (2020) 16:2204–14. 10.1080/21645515.2020.1790279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alley SJ, Stanton R, Browne M, To QG, Khalesi S, Williams SL, et al. As the pandemic progresses, how does willingness to vaccinate against COVID-19 evolve? Int J Environ Res Public Health. (2021) 18:2. 10.3390/ijerph18020797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Attwell K, Lake J, Sneddon J, Gerrans P, Blyth C, Lee J. Converting the maybes: Crucial for a successful COVID-19 vaccination strategy. PLoS ONE. (2021) 16:e0245907. 10.1371/journal.pone.0245907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bai W, Cai H, Liu S, Liu H, Qi H, Chen X, et al. Attitudes toward COVID-19 vaccines in Chinese college students. Int J Biol Sci. (2021) 17:1469–75. 10.7150/ijbs.58835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen M, Li Y, Chen J, Wen Z, Feng F, Zou H, et al. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum Vaccin Immunother. (2021) 17:2279–88. 10.1080/21645515.2020.1853449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Edwards B, Biddle N, Gray M, Sollis K. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLoS ONE. (2021) 16:e0248892. 10.1371/journal.pone.0248892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gan L, Chen Y, Hu P, Wu D, Zhu Y, Tan J, et al. Willingness to receive SARS-CoV-2 vaccination and associated factors among Chinese adults: a cross sectional survey. Int J Environ Res Public Health. (2021) 18:4. 10.3390/ijerph18041993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han K, Francis MR, Zhang R, Wang Q, Xia A, Lu L, et al. Confidence, acceptance and willingness to pay for the COVID-19 vaccine among migrants in Shanghai, China: a cross-sectional study. Vaccines. (2021) 9:5. 10.3390/vaccines9050443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huynh G, Nguyen TV, Nguyen DD, Lam QM, Pham TN, Nguyen HTN. Knowledge about COVID-19, beliefs and vaccination acceptance against COVID-19 among high-risk people in Ho Chi Minh City, Vietnam. Infect Drug Resist. (2021) 14:1773–80. 10.2147/IDR.S308446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kadoya Y, Watanapongvanich S, Yuktadatta P, Putthinun P, Lartey ST, Khan MSR. Willing or hesitant? a socioeconomic study on the potential acceptance of COVID-19 vaccine in Japan. Int J Environ Res Public Health. (2021) 18:9. 10.3390/ijerph18094864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kwok KO, Li KK, Wei WI, Tang A, Wong SYS, Lee SS. Editor's Choice: Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: a survey. Int J Nurs Stud. (2021) 114:103854. 10.1016/j.ijnurstu.2020.103854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin Y, Hu Z, Zhao Q, Alias H, Danaee M, Wong LP. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. (2020) 14:e0008961. 10.1371/journal.pntd.0008961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu D, Luo L, Xie F, Yu Z, Ma ZF, Wang Y, et al. Factors associated with the willingness and acceptance of SARS-CoV-2 vaccine from adult subjects in China. Hum Vaccin Immunother. (2021) 17:2405–14. 10.1080/21645515.2021.1899732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luk TT, Zhao S, Wu Y, Wong JY, Wang MP, Lam TH. Prevalence and determinants of SARS-CoV-2 vaccine hesitancy in Hong Kong: a population-based survey. Vaccine. (2021) 39:3602–7. 10.1016/j.vaccine.2021.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Machida M, Nakamura I, Kojima T, Saito R, Nakaya T, Hanibuchi T, et al. Acceptance of a COVID-19 vaccine in Japan during the COVID-19 pandemic. Vaccines. (2021) 9:3. 10.3390/vaccines9030210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qin W, Wang E, Ni Z. Chinese consumers' willingness to get a COVID-19 vaccine and willingness to pay for it. PLoS ONE. (2021) 16:e0250112. 10.1371/journal.pone.0250112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seale H, Heywood AE, Leask J, Sheel M, Durrheim DN, Bolsewicz K, et al. Examining Australian public perceptions and behaviors towards a future COVID-19 vaccine. BMC Infect Dis. (2021) 21:120. 10.1186/s12879-021-05833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sun Y, Chen X, Cao M, Xiang T, Zhang J, Wang P, et al. Will healthcare workers accept a COVID-19 vaccine when it becomes available? a cross-sectional study in China. Front Public Health. (2021) 9:664905. 10.3389/fpubh.2021.664905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tao L, Wang R, Liu J. Comparison of vaccine acceptance between COVID-19 and seasonal influenza among women in China: a national online survey based on health belief model. Front Med. (2021) 8:679520. 10.3389/fmed.2021.679520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thaker J. The persistence of vaccine hesitancy: COVID-19 vaccination intention in New Zealand. J Health Commun. (2021) 26:104–11. 10.1080/10810730.2021.1899346 [DOI] [PubMed] [Google Scholar]
- 168.Tsai FJ, Yang HW, Lin CP, Liu JZ. Acceptability of COVID-19 vaccines and protective behavior among adults in Taiwan: associations between risk perception and willingness to vaccinate against COVID-19. Int J Environ Res Public Health. (2021) 18:11. 10.3390/ijerph18115579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Walker AN, Zhang T, Peng XQ, Ge JJ, Gu H, You H. Vaccine acceptance and its influencing factors: an online cross-sectional study among international college students studying in China. Vaccines. (2021) 9:6. 10.3390/vaccines9060585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang C, Han B, Zhao T, Liu H, Liu B, Chen L, et al. Vaccination willingness, vaccine hesitancy, and estimated coverage at the first round of COVID-19 vaccination in China: a national cross-sectional study. Vaccine. (2021) 39:2833–42. 10.1016/j.vaccine.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang J, Lu X, Lai X, Lyu Y, Zhang H, Fenghuang Y, et al. The changing acceptance of COVID-19 vaccination in different epidemic phases in China: a longitudinal study. Vaccines. (2021) 9:3. 10.3390/vaccines9030191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang K, Wong EL, Ho KF, Cheung AW, Yau PS, Dong D, et al. Change of willingness to accept COVID-19 vaccine and reasons of vaccine hesitancy of working people at different waves of local epidemic in Hong Kong, China: repeated cross-sectional surveys. Vaccines. (2021) 9:1. 10.3390/vaccines9010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang K, Wong ELY, Ho KF, Cheung AWL, Chan EYY, Yeoh EK, et al. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. (2020) 38:7049–56. 10.1016/j.vaccine.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wong MCS, Wong ELY, Huang J, Cheung AWL, Law K, Chong MKC, et al. Acceptance of the COVID-19 vaccine based on the health belief model: a population-based survey in Hong Kong. Vaccine. (2021) 39:1148–56. 10.1016/j.vaccine.2020.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu B, Gao X, Zhang X, Hu Y, Yang H, Zhou YH. Real-world acceptance of COVID-19 vaccines among healthcare workers in perinatal medicine in China. Vaccines. (2021) 9:7. 10.3390/vaccines9070704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yan E, Lai DWL, Lee VWP. Predictors of intention to vaccinate against COVID-19 in the general public in Hong Kong: findings from a population-based, cross-sectional survey. Vaccines. (2021) 9:7. 10.3390/vaccines9070696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang F, Li X, Su X, Xiao T, Wang Y, Hu P, et al. A study on willingness and influencing factors to receive COVID-19 vaccination among Qingdao residents. Hum Vaccin Immunother. (2021) 17:408–13. 10.1080/21645515.2020.1817712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang KC, Fang Y, Cao H, Chen H, Hu T, Chen YQ, et al. Parental acceptability of COVID-19 vaccination for children under the age of 18 years: cross-sectional online survey. JMIR Pediatr Parent. (2020) 3:e24827. 10.2196/24827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang J, Jing R, Lai X, Zhang H, Lyu Y, Knoll MD, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. (2020) 8:3. 10.3390/vaccines8030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Riad A, Abdulqader H, Morgado M, Domnori S, Koščík M, Mendes JJ, et al. Global prevalence and drivers of dental students' COVID-19 vaccine hesitancy. Vaccines. (2021) 9:6. 10.3390/vaccines9060566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Skjefte M, Ngirbabul M, Akeju O, Escudero D, Hernandez-Diaz S, Wyszynski DF, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. (2021) 36:197–211. 10.1007/s10654-021-00728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akel KB, Masters NB, Shih SF, Lu Y, Wagner AL. Modification of a vaccine hesitancy scale for use in adult vaccinations in the United States and China. Hum Vaccin Immunother. (2021) 17:2639–46. 10.1080/21645515.2021.1884476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu T, He Z, Huang J, Yan N, Chen Q, Huang F, et al. A Comparison of vaccine hesitancy of COVID-19 vaccination in China and the United States. Vaccines. (2021) 9:6. 10.3390/vaccines9060649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Asadi Faezi N, Gholizadeh P, Sanogo M, Oumarou A, Mohamed MN, Cissoko Y, et al. Peoples' attitude toward COVID-19 vaccine, acceptance, and social trust among African and Middle East countries. Health Promot Perspect. (2021) 11:171–8. 10.34172/hpp.2021.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bono SA, Faria de Moura Villela E, Siau CS, Chen WS, Pengpid S, Hasan MT, et al. Factors affecting COVID-19 vaccine acceptance: an international survey among low- and middle-income countries. Vaccines. (2021) 9:5. 10.3390/vaccines9050515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Q, Yang L, Jin H, Lin L. Vaccination against COVID-19: a systematic review and meta-analysis of acceptability and its predictors. Prev Med. (2021) 150:106694. 10.1016/j.ypmed.2021.106694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. (2021) 9:2. 10.3390/vaccines9020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Al-Jayyousi GF, Sherbash MAM, Ali LAM, El-Heneidy A, Alhussaini NWZ, Elhassan MEA, et al. Factors influencing public attitudes towards COVID-19 vaccination: a scoping review informed by the socio-ecological model. Vaccines. (2021) 9:6. 10.3390/vaccines9060548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Al-Amer R, Maneze D, Everett B, Montayre J, Villarosa AR, Dwekat E, et al. COVID-19 vaccination intention in the first year of the pandemic: a systematic review. J Clin Nurs. (2021) 31:62–86. 10.1111/jocn.15951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. (2021) 194:245–51. 10.1016/j.puhe.2021.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lazarus JV, Wyka K, Rauh L, Rabin K, Ratzan S, Gostin LO, et al. Hesitant or not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: a country-level analysis. J Health Commun. (2020) 25:799–807. 10.1080/10810730.2020.1868630 [DOI] [PubMed] [Google Scholar]
- 192.Li M, Luo Y, Watson R, Zheng Y, Ren J, Tang J, et al. Healthcare workers' (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. (2021). [Epub ahead of print]. 10.1136/postgradmedj-2021-140195 [DOI] [PubMed] [Google Scholar]
- 193.Ullah I, Khan KS, Tahir MJ, Ahmed A, Harapan H. Myths and conspiracy theories on vaccines and COVID-19: potential effect on global vaccine refusals. Vacunas. (2021) 22:93–7. 10.1016/j.vacun.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wake AD. The Willingness to receive COVID-19 vaccine and its associated factors: “Vaccination refusal could prolong the war of this pandemic” - a systematic review. Risk Manag Healthc Policy. (2021) 14:2609–23. 10.2147/RMHP.S311074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brandstetter S, Böhmer MM, Pawellek M, Seelbach-Göbel B, Melter M, Kabesch M, et al. Parents' intention to get vaccinated and to have their child vaccinated against COVID-19: cross-sectional analyses using data from the KUNO-Kids health study. Eur J Pediatr. (2021) 180:3405–10. 10.1007/s00431-021-04094-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kaplan RM, Milstein A. Influence of a COVID-19 vaccine's effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. (2021) 118:10. 10.1073/pnas.2021726118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Harder T, Koch J, Vygen-Bonnet S, Külper-Schiek W, Pilic A, Reda S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Eurosurveillance. (2021) 26:2100563. 10.2807/1560-7917.ES.2021.26.28.2100563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jin Q, Raza S, Yousaf M, Zaman U, Siang J. Can communication strategies combat COVID-19 vaccine hesitancy with trade-off between public service messages and public skepticism? experimental evidence from Pakistan. Vaccines. (2021) 9:1–22. 10.3390/vaccines9070757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Goossen K, Hess S, Lunny C, Pieper D. Database combinations to retrieve systematic reviews in overviews of reviews: a methodological study. BMC Med Res Methodol. (2020) 20:138. 10.1186/s12874-020-00983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plots of vaccines acceptance.
Forest plots of vaccines acceptance by study designs.
Forest plots of vaccines acceptance by sampling methods.
Forest plots of vaccines acceptance by regions.
Forest plots of vaccines acceptance by population.
Forest plots of vaccines acceptance by gender.
Forest plots of vaccines acceptance by vaccines effectiveness.
Forest plots of vaccines acceptance by survey time.
Characteristics of the included studies.
J.B.I Critical appraisal checklist for studies reporting prevalence data.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.