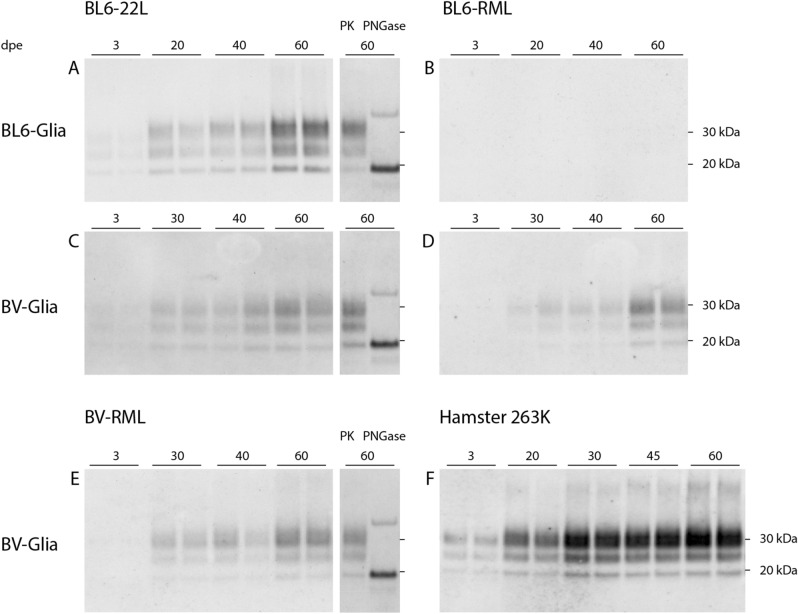
Figure 2.
Western blot analyses of PK-digested cell lysates of prion-infected glia cells from C57BL/6 mice and bank vole. Glia cells from mouse (A, B) or bank vole (C–F) were exposed to scrapie prion strains: mouse-adapted 22L (A, C), mouse-adapted RML (B, D), bank vole-adapted RML (E) and hamster-adapted 263K (F) for three days and harvested at the indicated time points. The immunoblots illustrate the propagation of pathological prion protein in cells over the cultivation time dependent on the seeding material. All four strains show an efficient propagation in bank vole glia cells, even for BL6-RML which shows no sign of infection in mouse cells up to 60 dpe. In A, C and E 60 dpe-samples deglycosylated with peptide-N-glycosidase F (PNGase F) are shown with a clear shift of the di- and monoglycosylated band to the unglycosylated band at 19 kDa. The band at ~35 kDa is considered an artefact of the deglycosylation process itself46. Representative Western blots of infection assays are shown, which were stained with anti-PrP antibody ICSM-18 (1:4000) (n = 3, except: 263K: n = 2; all in duplicates). The corresponding full-length Western blots are provided in the supplementary files p. 7–9 and exemplary for BV-RML blots of the three replicate experiments are shown on p. 6.