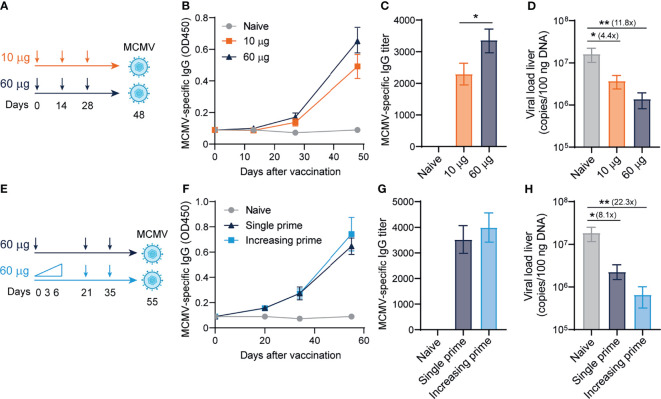
Figure 2.
Dose-escalating DNA-based vaccination improves antibody responses and viral protection. (A) Vaccination schedule for mice were vaccinated intradermally three times with different doses (10 ug and 60 ug) of the sgB DNA vaccine. Three weeks after the final vaccination, mice were challenged intraperitoneally with 5 × 104 PFU salivary gland-derived MCMV-Smith. (B) Kinetic analysis of the MCMV-specific IgG response in serum. Data shown are mean values ± SEM (n=8) (C) MCMV-specific endpoint binding IgG titers after three vaccinations. Data shown are mean values ± SEM (n=8). One-way ANOVA was used for statistical analysis. (D) At day 4 post-infection, livers were isolated and the viral genome copies were determined by PCR. The viral load is depicted as mean values ± SEM (n=8). (E) Vaccination schedule for mice vaccinated intradermally with either an increasing dose prime schedule (10 µg on day 0, 20 µg on day 3 and 30 µg on day 6) or a single prime dose of 60 µg on day 0, followed by two booster immunizations with a high dose (60 µg) of the sgB DNA vaccine. Three weeks after the final vaccination, mice were challenged intraperitoneally with 5 × 104 PFU salivary gland-derived MCMV-Smith. (F) Kinetic analysis of the MCMV-specific IgG response in serum. Data shown are mean values ± SEM (n=8) (G) MCMV-specific endpoint binding IgG titers after two booster vaccinations are shown and represent mean values ± SEM (n=8). One-way ANOVA was used for statistical analysis. (H) At day 4 post-infection, livers were isolated and the viral genome copies were determined by PCR. The viral load is depicted as mean values ± SEM (n=8). Experiments were performed twice with similar outcome. A Kruskal-Wallis test was used for statistical analysis. *P<0.05, **P<0.01.