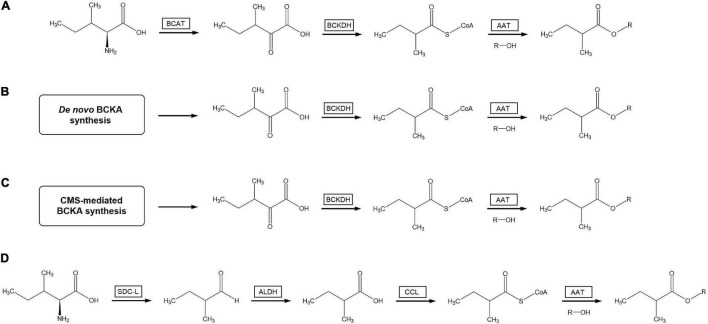
FIGURE 3.
Possible biosynthesis routes to volatile branched-chain acyl esters. (A) Mitochondrial BCAT- and BCKDH-mediated catabolism of free branched-chain amino acids to branched-chain acyl-CoAs followed by esterification with alcohols by AAT enzymes. (B) De novo chloroplast synthesis of branched-chain α-ketoacids followed by BCKDH-mediated catabolism to branched-chain acyl-CoAs and subsequent AAT-mediated esterification with alcohols. (C) CMS-initiated synthesis of α-keto-β-methylpentanoate followed by BCKDH-mediated catabolism to 2-methylbutyl acyl-CoA and subsequent AAT-mediated esterification with alcohols to form 2-methylbutanoate esters. (D) Branched-chain aldehyde synthesis from free branched-chain amino acids via SDC-L, followed by conversion of branched-chain aldehydes to branched-chain carboxylic acids through ALDH enzymes, followed by activation to branched-chain acyl-CoAs by CCL enzymes and subsequent condensation with alcohols via AAT to generate branched-chain acyl esters. The first, second, and fourth routes are capable of generating acyl esters with all three branched-chain structures, while the third route can only generate 2-methylbutanoate esters. The four pathways are illustrated yielding 2-methylbutanoate esters from appropriate precursors for ease of comparison. BCAT, branched-chain amino acid aminotransferase; BCKDH, branched-chain α-ketoacid dehydrogenase complex; AAT, alcohol acyltransferase; BCKA, branched-chain α-ketoacids; SDC-L, serine-decarboxylase like enzyme; ALDH, aldehyde dehydrogenase; CCL, carboxyl-CoA ligase.