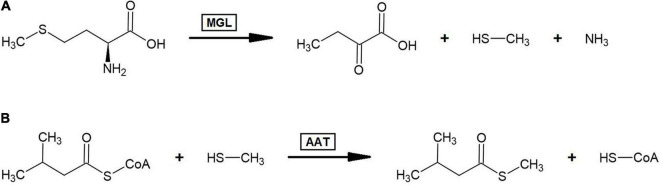
FIGURE 4.
Proposed biosynthesis pathway for S-methyl branched-chain thioester volatiles. (A) Breakdown of L-methionine to α-ketobutanoate, methanethiol, and ammonia by the action of L-methionine-γ–lyase (MGL). (B) Formation of S-methyl branched-chain thioesters via alcohol acyltransferase (AAT) mediated condensation of methanethiol with branched-chain acyl-CoAs. This panel illustrates this process occurring with 3-methylbutyl-CoA and yielding S-methyl 3-methylbutanethioate since that was the only S-methyl branched-chain thioester volatile identified across the 175 fruit volatile studies examined in this review; however, this process could theoretically yield S-methyl 2-methylpropanethioate and S-methyl 2-methylbutanethioate from 2-methylpropyl-CoA and 2-methylbutyl-CoA, respectively.