Figure 4.
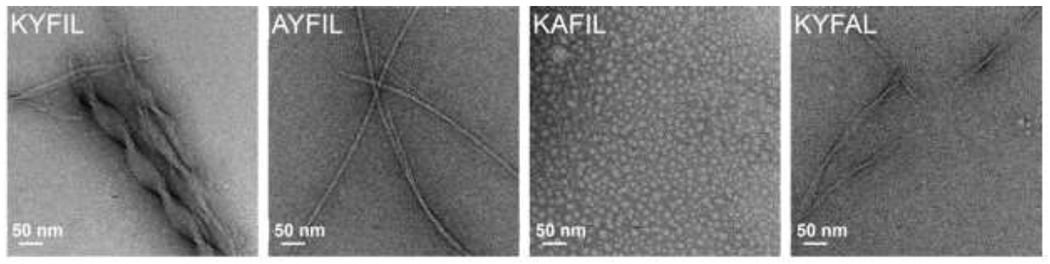
Sequence-dependent assembly of RAPID peptides (1.5 wt% in pH 7.4 PBS). TEM images of four different RAPID peptides highlight the sequence-dependent morphology. KYFIL and KYFAL assemble into hydrogels composed of twisted ribbons, while AYFIL assembles into hydrogels composed of twisted fibrils and KAFIL forms spherical nanostructures in solution without any hydrogel formation, likely because it is the only of these sequences that does not contain two adjacent aromatic residues. Further, the size of the ribbons formed differs by sequence, as KYFIL forms ribbons ca. 40 nm while KYFAL forms ribbons ca. 10 nm. Reproduced with permission from Tang et al. [84], Copyright (2019) American Chemical Society.
