Abstract
Objectives:
This study compared the prevalence of concentrated urine (urine specific gravity≥1.021), an indicator of hypohydration, across Tsimane’ hunter-forager-horticulturalists living in hot-humid lowland Bolivia and Daasanach agro-pastoralists living in hot-arid northern Kenya. It tested the hypotheses that household water and food insecurity would be associated with higher odds of hypohydration.
Methods:
This study collected spot urine samples and corresponding weather data along with data on household water and food insecurity, demographics, and health characteristics among 266 Tsimane’ households (N=224 men, 235 women, 219 children) and 136 Daasanach households (N=107 men, 120 women, 102 children).
Results:
The prevalence of hypohydration among Tsimane’ men (50.0%) and women (54.0%) was substantially higher (P<0.001) than for Daasanach men (15.9%) and women (17.5%); the prevalence of hypohydration among Tsimane’ (37.0%) and Daasanach (31.4%) children was not significantly different (P=0.33). Multiple logistic regression models suggested positive but not statistically significant trends between household water insecurity and odds of hypohydration within populations, yet some significant joint effects of water and food insecurity were observed. Heat index (2°C) was associated with a 23% (95%CI: 1.09–1.40, P=0.001), 34% (95%CI: 1.18–1.53, P<0.0005), and 23% (95%CI: 1.04–1.44, P=0.01) higher odds of hypohydration among Tsimane’ men, women, and children, respectively, and a 48% (95%CI: 1.02–2.15, P=0.04) increase in the odds among Daasanach women. Lactation status was also associated with hypohydration among Tsimane’ women (OR=3.43, 95%CI: 1.64–7.16, P=0.001).
Conclusion:
These results suggest that heat stress and reproductive status may have a greater impact on hydration status than water insecurity across diverse ecological contexts.
Keywords: Hydration, water insecurity, food insecurity, heat index, humidity
INTRODUCTION
Adequate hydration is critical for mammalian thermoregulation but is often difficult to maintain in hot environments. Humans, relative to other primates and mammals, have an exceptional ability to dissipate heat through sweating (Best et al., 2019). However, sweating can cause substantial body water loss (Sawka et al., 2001). In hot environments with scarce access to clean and acceptable sources of drinking water, it could be challenging to adequately replenish sweat-induced losses of body water. Dehydration resulting from water imbalances could in turn lead to a reduced sweat rate and thereby an increase in core body temperature, heart rate, and, potentially, heat related illness (Montain et al., 1995; Sawka et al., 2001; Sawka et al., 1985). Dehydration also puts stress on the kidneys and, if experienced repeatedly, could contribute to the onset and progression of chronic kidney disease (Johnson et al., 2014)
Rising temperatures worldwide appear to be contributing to an increasing risk of dehydration, heat stress, and kidney disease (Glaser et al., 2016; Johnson et al., 2019), and a projected 1 to 3 billion people may be inhabiting regions that in the next 50 years will regularly experience extreme mean annual temperatures of >29°C (Xu et al., 2020). These projections, combined with current and projected depletion of fresh water (Burek et al., 2016; Kjellstrom et al., 2016; Mekonnen & Hoekstra, 2016), means that the consequences of global warming for human well-being could be severe and beyond humans’ adaptive capacity (Xu et al., 2020). Already between 1996 and 2005, over 60 percent of the global population lived in areas with insufficient freshwater to meet population needs for one month out of the year, and an estimated 8 percent lived in areas where freshwater availability was insufficient year-round (Mekonnen & Hoekstra, 2016). Moreover, there are growing concerns about the safety and cleanliness of the water that is accessible (Chaplin-Kramer et al., 2019; UNICEF and WHO, 2017). For instance, fecal-contaminated water is known to be a major cause of diarrheal disease and mortality (Bain et al., 2014; Landrigan et al., 2018; World Health Organization, 2018), which in turn affects hydration status. With 10% of the global population still lacking access to basic drinking water services in 2017, and 29% lacking safely managed drinking water services (UNICEF and WHO, 2019), risk of drinking unclean water and suffering the resulting health consequences remains high in much of the world. Yet, research has not clearly established a direct relationship between water availability or quality and hydration status. Nor has there been a cross-culturally validated measure of overall water accessibility, adequacy, reliability, and safety, the collective components of water security, until recently (Young, Boateng, et al., 2019; Young, Collins, et al., 2019). There is, nevertheless, reason to believe that water insecurity would relate to risk of inadequate hydration (Rosinger, 2018).
Within a given level of water insecurity, there is likely further variation in the risk of dehydration in relation to weather patterns and physiological factors. For example, evaporative heat loss is less efficient in hot-humid climates relative to hot-arid climates (Best et al., 2019; Hanna & Tait, 2015; Mitchell et al., 1976). Dehydration risk may also vary by age and different life stages, as sweat rates of pre- and early pubertal children are lower than those of late-pubertal adolescents and adults (Falk et al., 1992; Meyer et al., 1992; Rivera-Brown et al., 1999). Furthermore, at advanced ages, renal capacity to concentrate urine in order to conserve body water may decline, at least in some populations (Hooper et al., 2014; Rosinger et al., 2019). Additionally, body composition (both fat stores and lean body mass) may influence fluid balance through its effects on thermoregulation and storage of body water (Kenney & Buskirk, 1995; Rosinger et al., 2016; Sawka et al., 2005). Finally, water needs are generally considered to be higher for pregnant and, especially, lactating women (European Food Safety Association, 2010; Institute of Medicine, 2005). All of these factors should be taken into account when assessing risk of inadequate hydration within water insecure contexts.
Food insecurity and intake of non-water beverages are also important factors to consider when exploring the relationship between water insecurity and hydration status. Moisture-rich foods (e.g., fruit, soup, porridge) may be important sources of hydration in water insecure regions (Rosinger & Tanner, 2015). However, food insecurity is closely tied to water insecurity (Brewis et al., 2019) and may further impact hydration through limited caloric intake or dietary diversity. Additionally, consumption of beverages made from boiled water (e.g., coffee or tea), non-water beverages (e.g., juice or milk), or fermented beverages (e.g., beer) (Rosinger & Bethancourt, 2020), may be preferable in areas where the water quality is questionable. Some of these beverages (e.g., those with added sugar or milk) may even increase water retention (Maughan et al., 2016; Maughan et al., 2019).
To better understand both inter- and intra-population variation in hydration status across two relatively extreme thermal environments, we sought to compare the prevalence and predictors of concentrated urine, a surrogate measure of hypohydration, among two populations experiencing different forms of water insecurity: 1) Tsimane’ forager-horticulturalists living in the hot-humid lowland, flood-prone Amazonian region of Bolivia, and 2) Daasanach semi-nomadic agro-pastoralists living in a hot-arid region of northern Kenya. Though Tsimane’ have ample access to water, their water sources are frequently contaminated with fecal bacteria (Rosinger, 2018). Daasanach, on the other hand, generally have extremely limited access to water of any quality and are, in fact, frequently in conflict with neighboring pastoralist groups over the limited water sources and grazing lands (Gebre, 2012).
The primary aim of this study was to compare the prevalence of hypohydration, as indicated by concentrated spot urine samples collected on a randomly sampled day and time, across the two study populations. We expected that there would be more cases of concentrated urine among Daasanach, for whom water is more difficult to access. The second aim was to test the hypotheses that household water insecurity and food insecurity would be positively related to concentrated urine, when controlling for heat index (a measure that incorporates ambient temperature and humidity), body mass index, and lactation and pregnancy status. The third and final aim was to test whether intake of non-water beverages (e.g., milk, sugar-sweetened beverages, and fermented beverages) might serve as culturally-specific buffers or nutritional adaptations to the extreme heat and water insecurity experienced in each site and be negatively associated with the odds of concentrated urine on a given day and time. Together, this work translates physiological responses to heat, humidity, reproductive costs, and water and food availability into lived experiences.
METHODS
Ethical Approval
This multisite study, called the Water Insecurity, Stress, and Hydration (WISH) Project, was conducted following the principles of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of Pennsylvania State University and the Kenya Medical Research Institute. Permission was also obtained from the Gran Consejo Tsimane’ in San Borja, Bolivia; the Director of Health in the county government of Marsabit, Kenya; and community leaders in each the five Tsimane’ communities sampled in Bolivia and each of the seven Daasanach communities sampled in Kenya. All participants provided oral informed consent.
Study populations and data collection
Tsimane’ study setting in Bolivia
Tsimane’ are a small-scale hunter-forager-horticultural population that inhabit a densely forested region of lowland Bolivia (150–300 m above sea level) at a latitude of ~14.10–15.40° S and 66.20–67.20°W (Figure 1) in the Beni Department. Average monthly temperatures recorded in San Borja, the nearest commercial town, have recently ranged over the last decade from ~20°C in the cooler months of June and July to 26–30°C in the warmer months of September and October (World Weather Online), and average annual temperatures have generally hovered around 26°C (Ruiz-Mallén et al., 2017). The annual lows in the region have ranged between 15°C and 17°C, while the annual highs have ranged between 31°C and 34°C (World Weather Online).
Figure 1.
Map of Tsimane’ and Daasanach Field Sites in Lowland Bolivia and Northern Kenya
Average monthly precipitation in the region has ranged in recent years from ~30–90 mm in the dry months of June-August to a range of 300–1000 mm in the wet months of December-March (World Weather Online). Average humidity rises with the rainfall during the wet months to ~80–90% but dips no lower than ~50–60% in the dry months (World Weather Online).
Tsimane’ consume a relatively high-carbohydrate, high-fiber, high-protein, low-fat diet consisting predominantly of cultivated starchy crops (e.g. plantain, rice, cassava, and maize) supplemented with lean game, freshwater fish and occasional fruit and honey (Gurven et al., 2017; Kraft et al., 2018). Their diets are supplemented episodically and to varying degrees with purchased wheat flour, pasta, bread, cooking oil, and sugar (Bethancourt et al., 2019; Kraft et al., 2018).
Access to modern amenities, such as running water, latrines, and electricity are extremely limited in Tsimane’ communities. The rivers along which most communities are built are a primary source of water, along with small streams, springs, ponds, and sometimes uncovered wells. Two out of the five communities sampled in this study had covered pump wells that had been constructed by an international organization within the previous decade. However, seven out of the ten pump wells in those two communities, in addition to the majority of the uncovered/unprotected water sources used in those and other communities, tested positive for hydrogen sulfide-producing bacteria. This bacteria (e.g., Salmonella, Klebsiella, Citrobacter, Clostridium, and others) is potentially pathogenic, and their presence suggests the water sources are contaminated with fecal content (unpublished work).
There is substantial variation within and across Tsimane’ communities in access to modern market economies, so consumption of purchased drinks is only episodic. Among Tsimane’ communities in general, however, there has been a gradual increase in recent decades in the purchase and consumption of sugar (Bethancourt et al., 2019; Kraft et al., 2018), which is often added to water, either by itself or in combination with fruit. Tsimane’ also have a history of making a beverage from cassava, plantain, or (occasionally) maize, called “chicha”, which they sometimes allow to ferment for several days. Chicha has traditionally been an important source of hydration among Tsimane’ communities (Rosinger & Tanner, 2015), but chicha consumption has been declining in recent decades (Rosinger & Bethancourt, 2020).
Daasanach study setting in Kenya
Daasanach in Kenya are semi-nomadic agro-pastoralists. Permanent Daasanach settlements surround the town of Ileret located at 4.314° N, 36.227° E (Figure 1) and ~380 m above sea level in on the northeastern shore of Lake Turkana in northern Kenya, and nomadic Daasanach communities tend to move around regions south of Ileret and north into Ethiopia. Six of the seven communities we sampled were relatively permanent, with many houses in place for months or years. One community was nomadic, with residents staying in one location for only days or weeks at a time.
Over the last decade, average monthly temperatures in Lodwar, the nearest weather station located on the opposite (southwest) side of Lake Turkana, have ranged from 27°C to 34°C over the last decade (World Weather Online). Annual lows ranged between 22°C and 27°C, and highs ranged between 35°C and 37°C (World Weather Online).
This region experiences two rainy seasons that generally occur between March and May and again between October and December. Data from the Lodwar weather station suggests that the region received a range of 54 to 725 mm of rainfall annually between 1950 and 2012, with an average of 217 mm per year (Opiyo et al., 2014). However, peak monthly precipitation over the last decade never surpassed 110 mm, and some months passed with <1 mm of rain (World Weather Online). These figures may even overestimate levels of precipitation on the northeastern side of Lake Turkana, as Lodwar may receive more rain when moisture blowing from the northeast toward the southeast hits the Loima Hills in the west.
While agriculture may be practiced more regularly by Daasanach living in closer proximity to the Omo River in Ethiopia (Gebre, 2012), agricultural activity appears to be less common or more sporadic in the Lake Turkana region of northern Kenya due to lack of water or irrigation. The diets of Daasanach in our study sample comprised primarily maize, beans, and sorghum that they obtained at small stores or from passing merchants, along with milk and meat from their goats, cattle, and (occasionally) camels. Consumption of meat, however, is often either opportunistic (e.g., an animal dies or is killed) or reserved for ceremonies and celebrations.
As in Tsimane’ communities, access to piped water, sanitation, and electricity are rare among Daasanach households. Nine of the ten reported drinking water sources we tested were positive for hydrogen sulfide-producing bacteria (unpublished data). Only one household reported using collected rainwater, and the nomadic community was, at the time, collecting their water from both a distant pond and a remote borehole. However, most households we sampled relied on water collected from holes dug in dry riverbeds. Due to concerns about the salinity and fluoride levels of Lake Turkana (Serem, 2012; Yuretich & Cerling, 1983), the lake is reportedly rarely used as a source of drinking water.
Households could generally find water within one km from their home, though the nomadic community was traveling nearly 3–4 km to collect water. We observed some water sources to be crowded at certain times of the day, as they were shared by people, livestock, and wild animals. Hence, the process of traveling to a water source and waiting one’s turn to collect water was often reported to amount to a two- to three-hour round-trip affair. Moreover, families often had a limited number of storage containers with which to carry water, so often at least two, and sometimes three, trips had to be made each day, generally split among different females in the household.
Daasanach have a daily (often two or three times/day) custom of drinking tea made with black tea leaves or coffee made with coffee husks. If available, they will add milk and sugar to their tea or coffee. Milk is not available or consumed as frequently among Daasanach living close to Ileret, as the livestock often have to remain in distant locations where there is more pasture. Daasanach will occasionally (primarily for ceremonies) make a fermented beverage with millet, called “mutukuru.” More recently, however, it is more common to make a more highly alcoholic beverage, called “kada,” by combining brewer’s yeast, sugar, and water, a process that is substantially less time consuming than making millet beer. Distilled liquor can also occasionally be purchased from some shopkeepers or passing merchants.
Participant Recruitment
In Bolivia, we attempted exhaustive sampling as all households in the five sampled communities were invited to participate in the study. We had approximately a >90% survey participation rate in all communities, but approximately 8% of men, 11% of women, and 15% of children surveyed declined to provide urine samples.
In Kenya, due to fewer days available to spend in each community, we worked with local elders, community health assistants, and community health volunteers to recruit members from every third house (and moving to the house next door if the family was not home) within the seven sampled communities. We obtained a feasibility sample in each community based on the number of families we could survey in the pre-specified number of days allocated to spend in each community, with a range of 12 to 28 households sampled per community.
In both study sites, we sought to survey the male and female head of the household and two children, ideally male and female, between the ages of 6 and 16. We were not always successful at sampling two children from each household, either because some households did not have two children within that age range or because children were in school (in Bolivia) or out tending to the livestock (in Kenya).
Data collection
Data were collected after the end of the rainy season in both lowland Bolivia (April and May 2019) and northern Kenya (June and July 2019). In both study locations, an approximately 60-minute survey was administered to each household. Surveys were conducted in the respective local languages by two Bolivian researchers with the help of two Tsimane’ translators in Bolivia and two American researchers with the help of three Daasanach translators in Kenya. Household members were surveyed together; parents answered for children when children were unwilling to answer for themselves. Anthropometric data and urine samples were generally collected following the survey in Bolivia and prior to the survey in Kenya. Study procedures were consistent across sites.
Outcome: hydration status
Each member of the household was asked to provide a spot urine sample to measure acute hydration status using urine specific gravity (USG), the ratio of the density of urine (g/mL) to the density of water (Armstrong, 2007). As with spot urine osmolality (Stookey et al., 2020), spot USG is sensitive to acute changes in fluid intake and excretion and is, therefore not a reliable measure of “usual” hydration status. However, compared with serum-based measures of hydration, which are less responsive to acute external influences on water balance, USG provides a better picture of how hydration status at a given time relates to fluctuations in environmental factors, such as temperature and humidity.
Urine samples were collected, covered, and set aside to cool for 10–30 minutes in the shade before USG was measured in triplicate using a digital refractometer (Atago) after calibration in bottled or distilled water. Concentrated urine, our indicator of hypohydration, was defined as USG≥1.021 (Armstrong et al., 2010; Cheuvront et al., 2010). We also examined the proportion of individuals with USG≥1.025 and USG>1.027, which may provide a categorical index of more severe levels of dehydration (Armstrong et al., 2010; Cheuvront et al., 2016).
Predictors of interest
Household water insecurity and access
Household water insecurity was measured using the 12-item Household Water InSecurity Experience (HWISE) Scale, which has been validated for quantifying experiences of water access, use, and reliability in low- and middle-income populations (Young, Boateng, et al., 2019; Young, Collins, et al., 2019). Households were asked about the frequency they experienced 12 different phenomena related to water insecurity in the prior four weeks (e.g. not having enough to drink, changing what was being cooked because of problems with water, or going to sleep thirsty; see Supplemental Information for the full list of questions). A score of 0, 1, 2, or 3 was given to answers of never, rarely, sometimes, or often/always, respectively, for a maximum of 36 points for all 12 questions. A score of ≥12 is considered at least moderately water insecure (Young, Boateng, et al., 2019). Households were also asked how many minutes a single trip to their water source, including wait time, generally took; this served as a measure of both household distance from a water source and the relative burden of water collection.
Household food insecurity
Household food insecurity was measured using the 9-item Household Food Insecurity Access Scale (HFIAS) (Coates et al., 2007). The HFIAS module asks about the frequency in the previous four weeks that the household experienced 9 different phenomena related to food insecurity (e.g. worried about having enough food, skipping meals, or having limited food variety; see Supplemental Information for the full list of questions). A score of 0, 1, 2, or 3 was given to answers of never, rarely, sometimes, or often/always, respectively, for a maximum score of 27. Moderate and severe food insecurity categorization was assessed based on the number of affirmative responses to more extreme food access questions (e.g., frequency of going an entire day or night without food) (Coates et al., 2007)
To ascertain a proxy of protein intake, a surrogate measure of renal solute load, adults of each household were asked about the number of times in the previous week that they had consumed any kind of meat, poultry, or fish.
Consumption of non-water beverages
During the surveys, each member of the household reported the number of days in the previous week they had consumed a variety of non-water beverages locally available to them. In Bolivia, this included soda; juice; water mixed with plain sugar, sweetened flavoring, or fruit; unfermented chicha (homemade from mixing cooked and mashed or masticated cassava, plantain, or maize with water); fermented chicha (chicha allowed to sit out for several days and become mildly alcoholic); and liquor. In Kenya, this included soda, juice, tea or coffee mixed with sugar, milk, homemade beer (either made with sugar and yeast or fermented millet), bottled beer, and liquor. For tea and coffee we asked Daasanach about the number times per day (as opposed to number of days per week) those beverages were consumed. If milk was consumed, we asked whether it was consumed several times per week, daily, or multiple times per day.
We created binary variables to indicate whether participants had consumed any beverages with added sugar, unfermented chicha, homemade fermented beverages, liquor, or any beverages with any amount of alcohol in the previous week. For milk, we created a binary variable indicating whether it was consumed daily or less than daily.
Heat index
Ambient temperature and humidity at the time of the urine sample collection were measured in Bolivia using a mercury thermometer and a Fischer hair hygrometer and in Kenya using a digital weather meter (Kestrel Instruments 3000). Heat index for Bolivia was calculated manually from temperature and humidity measures using the Rothfusz equation (Anderson et al., 2013; NOAA National Weather Service Weather Prediction Center, 2014; Rothfusz, 1990); for Kenya, calculated heat index measures were provided by the Kestrel digital weather meter. While heat index is a perceptual index, it was used here as a surrogate measure of environmental heat stress due to the absence of air movement and solar radiation data.
Anthropometrics
Standing height without shoes was measured using a SECA stadiometer on a hard, flat surface. Weight and body fat percentage were measured using a Tanita BF-680W bioelectric impedance scale. BMI was calculated as kg/m2. Fat-free mass (FFM) was estimated as [weight-(weight * body fat percentage). The Tanita scale also calculates total body water for individuals whose age is entered into as ≥18; the age of 18 was typically imputed for study participants age ≥16 years. Total body water was not measured for children <16 years.
BMI-for-age z-scores were calculated for children <16 years using the World Health Organization reference values and macros available on their website (World Health Organization).
Demographics
Demographic data were collected during the survey. Many adults in Tsimane’ and Daasanach communities do not know their birth dates and, therefore, do not know their exact age. Because of recent government efforts to provide identification cards, some adults have identification cards on which a birth date is given. For this study, age was estimated by either using the birth date provided on an identification card or by probing about when the participant was born in relation to locally-known historical events. Birth dates for children were sometimes better documented and, if not, were estimated from dental eruption and by asking about the number of years they had received formal schooling. We also collected information on whether individuals were born in the rainy or dry season to explore the potential relationship between early childhood environments and odds of hypohydration.
Statistical analyses
All analyses were performed using Stata version 15.1 (Statacorp, College Station, TX). For our primary aim, chi-squared tests were used to compare the prevalence of USG≥1.021 across Tsimane’ and Daasanach men, women, and children. As a higher proportion of Tsimane’ households were sampled in the afternoon, we repeated these tests for just the individuals in population that were sampled in the morning hours (before noon).
For our secondary aim, multiple logistic regression models with robust standard errors clustered on household were used to test the odds of USG≥1.021 in relation to water and food insecurity scores among adult males, adult females (≥16 years old), and children (each tested separately). Two females reported to be 15 years old were included in the ≥16 year old group because they were pregnant or lactating. We began by setting up a baseline model which included all available covariates we decided a priori were important physiological or weather-related variables associated with variation in hydration status: age, BMI (or BMI-for-age z-score for children), heat index (in 2 degree Celsius increments), an indicator of whether the urine sample was collected in the morning (before noon) or afternoon, and time required to fetch water (in 10 minute increments). Though the VIFs for time of urine collection and heat index were <3 for all subsamples, we modeled time of day as a binary rather than continuous variable to avoid issues of multicollinarity. The baseline model for adult females also included a categorical variable indicating whether they were pregnant, lactating, or both.
To test our hypotheses, the HWISE water insecurity score was first added as a continuous variable to the baseline model. The continuous variable for the HFIAS food insecurity score was then added to the model with the water insecurity score to test for both the independent effects of water and food insecurity and their joint effects using the “lincom” post-estimation command in Stata. Finally, the full model tested for both main and interactive effects of water and food insecurity with post-estimation tests of the joint effects of the water and food insecurity scores and their interaction term.
We also performed sensitivity baseline analyses that replaced BMI (or BMI-for-age z-score) with bioelectric impedance measured body fat percentage or calculated FFM. Another sensitivity model added birth season to the baseline model to explore whether there was any relationship between the odds of hypohydration and having been born in the wet or dry season. Furthermore, because USG measures may be impacted by high protein intake (Martin et al., 2006), we performed a sensitivity analysis that added reported frequency (number of times in the previous week) of consuming meat, poultry, or fish. In a final sensitivity analysis, we added an indicator of whether or not the individual had reported either diarrhea or fever in the previous week. The results of each of the aforementioned models and sensitivity tests, along with goodness-of-fit measures, can be found in Supplemental Tables 1–6.
Finally, to test associations of recent non-water beverage intake and hydration status, we examined whether the odds of concentrated urine were associated with intake of any sugar- or fruit-sweetened beverages, unfermented chicha (for Tsimane’), homemade fermented beverages, liquor, or any alcohol beverages when controlling for baseline variables. For Daasanach we also tested whether daily milk intake or times per day of tea or coffee consumption were associated with the odds of concentrated urine.
Analytic sample
After excluding 20 men, 30 women, and 40 children who did not provide urine samples and one woman with missing data on reproductive status, our Tsimane’ sample included a total of 224 men and 235 women ≥16 years and 219 children <16 years from 266 Tsimane’ households. After excluding one man, one woman, and 10 children who did not provide urine samples, along with 14 women with missing data at random on reproductive status, our Daasanach sample included 107 men and 120 women ≥16 years and 102 children <16 years from 136 households. Tsimane’ children with missing data tended to be slightly older by an average of 0.8 years (P=0.05) while Daasanach children with missing data tended by be slightly younger by an average of 1.9 years (P=0.03); otherwise, those with and without missing data were comparable in age and BMI.
All analyses were estimated separately for Tsimane’ and Daasanach subsamples because different instruments were used for measuring temperature and humidity in the two sites. Adults were modeled separately from children <16 years old because of known differences in sweat rates between adults and pre- and early-pubertal children (Falk et al., 1992; Meyer et al., 1992; Rivera-Brown et al., 1999) and different water needs based on female reproductive status.
RESULTS
Household-level characteristics and weather measures
Differences in water access and water insecurity between the two populations were remarkable. The average roundtrip time to fetch water was ~2 hours for Daasanach compared with ~7 minutes for Tsimane’ (Table 1). Approximately 93.4% of Daasanach households had water insecurity scores suggestive of moderate to severe water insecurity, but few Tsimane’ households reported problems obtaining adequate water to meet their needs. Average food insecurity was likewise lower among Tsimane’ households compared with Daasanach households.
Table 1.
Household and Environmental Characteristics of study populations
| Tsimane' Households (N=266) | Daasanach Households (N=136) | |||
|---|---|---|---|---|
| Mean or % |
(Min, Max) |
Mean or % |
(Min, Max) |
|
| Roundtrip time to fetch water (minutes) | 7.0 | (1.0, 60.0) | 121.0 | (1.0, 240.0) |
| HWISE Score (0–36) | 2.1 | (0, 20.0) | 20.2 | (0, 36.0) |
| Moderate or severe water insecurity (%) | 1.9% | 93.4% | ||
| HFIAS Score (0–27) | 6.4 | (0, 18.0) | 17.3 | (0, 27.0) |
| Moderate or severe food insecurity (%) | 87.2% | 98.5% | ||
| Ambient Temperature (C°) | 28.1 | (18.0, 33.0) | 32.3 | (21.6, 38.8) |
| Relative Humidity (Percent) | 76.9 | (53.0, 96.0) | 47.5 | (25.4, 85.6) |
| Heat Index (C°) | 32.2 | (18.1, 39.7) | 34.2 | (21.4, 45.0) |
Though the average ambient temperature in lowland Bolivia (28.1°C) at the time of data collection was lower than in northern Kenya (32.3°C), the average relative humidity was substantially higher in Bolivia (76.9%) compared to Kenya (47.5%). Consequently, the heat index was similar, on average, in the Bolivia (32.2°C) and Kenya (34.2°C) field sites.
Sample demographic and health characteristics
The Tsimane’ and Daasanach adult and children subsamples were similar in terms of age (Tables 2a and 2b). There was a higher proportion of females (60.8% vs 47.9%) among the Daasanach children sampled. A similar proportion of Tsimane’ (52.8%) and Daasanach (50.8%) women were pregnant and/or lactating.
Table 2a.
Demographic and Health Characteristics of Tsimane’ and Daasanach Adult Participants
| Tsimane' |
Daasanach |
|||||||
|---|---|---|---|---|---|---|---|---|
| Men (n=224) | Women (n=235) | Men (n=107) | Women (n=120) | |||||
| Mean or % | (Min, Max) | Mean or % | (Min, Max) | Mean or % | (Min, Max) | Mean or % | (Min, Max) | |
|
| ||||||||
| Age (years) | 39.5 | (16.0, 90.0) | 34.5 | (15.0, 98.0) | 46.1 | (16.0, 79.0) | 34.3 | (18.0, 78.0) |
| Bom in wet season (%)† | 46.7% | 46.4% | 35.5% | 29.2% | ||||
| Lactating (%) | 37.9% | 38.3% | ||||||
| Pregnant (%) | 10.6% | 12.5% | ||||||
| Pregnant & Lactating (%) | 4.3% | 0% | ||||||
| W eight (kg)† | 63.1 | (44.9, 87.1) | 55.2 | (19.1, 83.1) | 52.8 | (34.1, 96.8) | 49.4 | (34.5, 91.3) |
| Height (cm) | 162.5 | (151.2, 178.5) | 150.6 | (113.0, 165.5) | 172.5 | (154.1, 186.8) | 163.8 | (145.8, 180.7) |
| BMI (kg/m2)‡ | 23.9 | (19.6, 31.3) | 24.3 | (15.0, 38.5) | 17.7 | (13.9, 29.8) | 18.5 | (12.7, 37.1) |
| BMI <18.5 kg/m2‡ | 0% | 3.0% | 74.8% | 58.1% | ||||
| BMI ≥25 kg/m2† | 25.4% | 39.5% | 0.9% | 3.8% | ||||
| Body fat percentageठ| 17.3 | (9.6, 32.9) | 26.1 | (7.3, 43.5) | 9.7 | (2.9, 30.9) | 21.8 | (3.8, 41.7) |
| Fat-free massठ| 52.0 | (38.0, 70.3) | 40.2 | (15.7, 54.7) | 47.5 | (32.3, 66.9) | 38.1 | (31.0, 58.2) |
| Total body water percentage¶ | 59.7 | (47.6, 69.4) | 50.9 | (39.5, 62.9) | 61.7 | (46.9, 69.6) | 53.3 | (40.4, 65.1) |
| Afternoon urine collection (%) | 52.7% | 54.9% | 31.8% | 31.7% | ||||
| USG | 1.020 | (1.004, 1.036) | 1.021 | (1.002, 1.038) | 1.011 | (1.000, 1.033) | 1.011 | (1.000, 1.030) |
| USG≥1.021 (%) | 50.0% | 54.0% | 15.9% | 17.5% | ||||
| USG≥1.025 (%) | 22.8% | 32.8% | 7.5% | 10.0% | ||||
| USG>1.027 (%) | 10.7% | 17.9% | 1.9% | 5.0% | ||||
| Diarrhea in last week (%) | 1.8% | 1.3% | 2.8% | 7.5% | ||||
| Fever in last week (%) | 0.9% | 0.9% | 7.5% | 9.2% | ||||
| Beverages and food in previous week | ||||||||
| Any sugar drinks (%) | 84.8% | 85.1% | 70.1% | 67.5% | ||||
| Any unfermented chicha (%) | 64.7% | 63.4% | - | - | ||||
| No milk (%) | - | - | 45.8% | 39.2% | ||||
| Milk < daily (%) | - | - | 20.6% | 21.7% | ||||
| Milk daily (%) | - | - | 33.6% | 39.2% | ||||
| Times/day of tea or coffee | - | - | 2.1 | (0, 4.0) | 2.1 | (0, 5) | ||
| Any homemade fermented beverage (%) †† | 15.6% | 3.0% | 34.0% | 16.7% | ||||
| Any bottled beer (%) | - | - | 8.5% | 0.8% | ||||
| Any liquor (%)‡‡ | 19.4% | 0.4% | 15.1% | 7.5% | ||||
| Any alcohol (%) | 23.2% | 3.4% | 41.5% | 20.8% | ||||
| Days of consuming meat or fish in the previous last week§§ | 5.9 | (1, 7) | 5.7 | (0, 7) | 2.2 | (0, 7.0) | 2.1 | (0, 7) |
Missing data for 27 Tsimane’ adult males and 22 Tsimane’ adult females.
Excluding measures from 34 pregnant Tsimane’ 15 pregnant Daasanach women.
Missing data for 3 Daasanach adult males and 1 Daasanach adult female.
Missing data for 2 Tsimane’ adult males, 1 Tsimane’ adult female, 3 Daasanach adult males, and 1 Daasanach adult female.
Missing data for 1 Tsimane adult female and 1 Daasanach adult male.
Missing data for 2 Tsimane’ adult males and 1 Daasanach adult male.
Missing data for 3 Tsimane’ adult males, 4 Tsimane’ adult females, 1 Daasanach adult male, and 4 Daasanach adult females.
Table 2b.
Demographic and Health Characteristics of Tsimane’ and Daasanach Child Participants
| Tsimane' |
Daasanach |
|||
|---|---|---|---|---|
| Children (n=219) | Children (n=102) | |||
| Mean or % | (Min, Max) | Mean or % | (Min, Max) | |
|
| ||||
| Male (%) | 52.1% | 39.2% | ||
| Age (years) | 9.7 | (6.0, 15.0) | 9.3 | (6.0, 15.0) |
| Bom in wet season (%)† | 46.8% | 39.2% | ||
| Weight (kg) | 29.9 | (16.2, 65.3) | 23.1 | (13.8, 49.5) |
| Height (cm) | 129.0 | (103.6, 168.8) | 128.6 | (105.1, 175.5) |
| BMI-for-age z-score | 0.3 | (−1.6, 1.9) | −2.1 | (−4.4, 0.1) |
| BMI z-score < -2 SD | 0% | 53.9% | ||
| BMI z-score > 1 SD | 17.8% | 0% | ||
| Body fat percentage‡ | 18.9 | (8.9, 36.4) | 13.4 | (2.3, 22.9) |
| Fat-free mass‡ | 24.1 | (13.5, 55.6) | 20.2 | (11.9, 45.0) |
| Afternoon urine collection (%) | 59.8% | 27.5% | ||
| Average USG | 1.018 | (1.002, 1.035) | 1.016 | (1.0002, 1.034) |
| USG≥1.021 (%) | 37.0% | 31.4% | ||
| USG≥1.025 (%) | 15.1% | 19.6% | ||
| USG>1.027 (%) | 7.8% | 11.8% | ||
| Diarrhea in last week (%) | 1.8% | 3.9% | ||
| Fever in last week (%) | 3.2% | 11.8% | ||
| Beverages in the previous week | ||||
| Any sugar drinks (%) | 81.3% | 63.7% | ||
| Any unfermented chicha (%)§ | 60.9% | - | ||
| No milk (%) | - | 39.2% | ||
| Milk < daily (%) | - | 25.5% | ||
| Milk daily (%) | - | 35.3% | ||
| Times/day of tea or coffee | - | 0.6 | (0, 1.0) | |
Missing data for 31 Tsimane’ children.
Missing data for 4 Daasanach children.
Missing data for 4 Tsimane’ children.
There were substantial differences across the two study populations in height and body composition. Non-pregnant Tsimane’ adults were, on average, shorter but heavier than Daasanach adults. The prevalence of underweight (classified as <18.5 kg/m2 (National Institutes of Health, 1998)) was high among Daasanach men (74.8%) and women (58.1%), whereas only 3.0% of Tsimane’ women and no Tsimane’ men were underweight. In contrast, 25.4% and 39.5% of Tsimane’ men and non-pregnant women had a BMI≥25 kg/m2, while only 0.9% of men and 3.8% of non-pregnant Daasanach women in our sample had BMI≥25 kg/m2. Likewise, 53.9% of Daasanach children but no Tsimane’ children were considered underweight (BMI-for-age z-score<−2 SD (World Health Organization, 2010)), while 17.8% of Tsimane’ children and no Daasanach children were considered overweight (BMI-for-age z-score>1 SD (World Health Organization, 2010)).
The majority of adults reported consuming at least one beverage with sugar in the previous week, most often in the form of tea or coffee (for Daasanach) or plain water with sugar added (for Tsimane’). Only 36.6% of Daasanach adults reported daily milk consumption in the previous week, and 42.3% reported no recent milk intake. Characteristics of non-alcoholic beverage consumption among Tsimane’ and Daasanach children followed the patterns of adults.
In both populations, alcohol consumption was substantially higher among men than women (23.2% versus 3.4% among Tsimane’ and 41.5% versus 20.8% among Daasanach), but it was higher among both men and women in Daasanach communities compared with Tsimane’. Homemade fermented brews were more common than liquor among Daasanach, but this was not the case for Tsimane’.
Primary Aim: comparison of hydration status among Tsimane’ living in a hot-humid lowland Bolivia and Daasanach living in a hot-arid northern Kenya
We expected Daasanach would have more concentrated urine and be more likely to be hypohydrated. However, average USG was significantly higher among Tsimane’ men and women (1.020 and 1.021) than among Daasanach men and women (both 1.011) (Table 2a). In contrast to our expectations, the prevalence of USG ≥1.021 was significantly higher among Tsimane’ adults compared with Daasanach adults, as 50.0% and 54.0% of Tsimane men and women were hypohydrated at the time of urine collection compared with 15.9% and 17.5% of Daasanach men and women (P<0.0005). A higher prevalence of concentrated urine among Tsimane’ men (42.5% vs 12.3%, P<0.0005) and women (50.9% vs 9.8%, P<0.0005) relative to Daasanach men and women was observed even when restricting the comparison to participants sampled before noon (data not shown). Sex differences were found among Tsimane’, as more women than men had USG measures in the upper thresholds of 1.025 (32.8% vs 22.8%, P=0.02) and 1.027 (17.9% vs 10.7%, P=0.01). There was no significant difference in the proportion of Daasanach men and women with USG in those upper ranges.
Compared with adults, Tsimane’ and Daasanach children were similar in the proportions with concentrated urine (Table 2b). Approximately 37.0% of Tsimane’ children and 31.4% of Daasanach children had USG≥1.021 (P=0.33). Very concentrated urine (USG>1.027) was identified in 7.8% and 11.8% of Tsimane’ and Daasanach children, respectively.
Second Aim: Hydration status in relation to household water and food insecurity
In contrast to our hypotheses, the full multiple logistic regression models that included main and interactive effects for water and food insecurity scores, in addition to the baseline control variables, did not provide evidence for a significant independent association between water insecurity and odds of hypohydration within either population, though the coefficients for water insecurity were all positive (Table 3; Figures 2). Food insecurity was likewise not a significant independent predictor of the odds of concentrated urine (Figure 3), though a positive trend nearing significance was observed for Tsimane’ children and Daasanach women. However, we observed significant joint effects of water and food insecurity among Tsimane’ children (OR: 1.17, 95% CI: 1.00–1.36, P=0.048) in the model that included scores for HWISE and HFIAS without an interaction (data not shown). Likewise, significant joint effects were observed among Daasanach women (OR: 1.35, 95% CI: 1.02–1.80, P=0.037) in the model that included the interaction between HWISE and HFIAS (data not shown).
Table 3.
Multiple logistic regression analyses: odds of concentrated urine (USG≥1.021) in relation to physiological and environmental factors and water and food insecurity
| Tsimane' |
Daasanach |
|||||
|---|---|---|---|---|---|---|
| Men (n=224) |
Women (n=235) |
Children (n=219) |
Men (n=108) |
Women (n=120) |
Children (n=102) |
|
| OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
| HWISE score (1-pt increments) | 1.15 (0.86 – 1.55) | 1.21 (0.88 – 1.65) | 1.28 (0.84 – 1.95) | 1.07 (0.92 – 1.25) | 1.14 (0.96 – 1.37) | 1.05 (0.83 – 1.34) |
| HFIAS score (1-pt increments) | 1.01 (0.92 – 1.10) | 0.97 (0.89 – 1.06) | 1.10 (1.00 – 1.21) | 0.96 (0.83 – 1.11) | 1.19 (1.00 – 1.43) | 1.10 (0.89 – 1.36) |
| HWISE × HFIAS interaction | 0.99 (0.96 – 1.01) | 0.99 (0.96 – 1.02) | 0.98 (0.94 – 1.02) | 1.00 (0.99 – 1.01) | 0.99 (0.98 – 1.00) | 1.00 (0.99 – 1.01) |
| Heat Index (2 degree increments, C°) | 1 23*** (1.09 – 1.40) | 134*** (1.18 – 1.53) | 1.23* (1.04 – 1.44) | 1.10 (0.78 – 1.55) | 1.48* (1.02 – 2.15) | 0.98 (0.69 – 1.38) |
| Afternoon sample | 1.42 (0.80 – 2.53) | 1.00 (0.56 – 1.80) | 1.63 (0.82 – 3.22) | 1.65 (0.44 – 6.19) | 2.52 (0.56 – 11.36) | 9.41*** (2.68 – 32.98) |
| BMI (kg/m2) | 1.10 (0.97 – 1.26) | 1.06 (0.98 – 1.15) | 0.95 (0.58 – 1.54) | 0.80 (0.54 – 1.19) | 1.32** (1.10 – 1.59) | 1.21 (0.68 – 2.14) |
| Individual's age (year increments) | 1.01 (0.99 – 1.03) | 1.03* (1.00 – 1.05) | 1.10 (0.98 – 1.24) | 1.01 (0.97 – 1.04) | 0.93 (0.87 – 1.00) | 1.10 (0.93 – 1.30) |
| Time to collect water (10 min increments) | 0.91 (0.61 – 1.36) | 1.15 (0.81 – 1.64) | 1.59 (0.82 – 3.09) | 1.00 (0.87 – 1.13) | 0.90 (0.78 – 1.04) | 0.94 (0.86 – 1.02) |
| Lactating | 3.35** (1.62 – 6.95) | 1.93 (0.49 – 7.68) | ||||
| Pregnant | 0.58 (0.22 – 1.56) | 2.30 (0.34 – 15.77) | ||||
| Pregnant & lactating | 1.28 (0.37 – 4.44) | |||||
| Male | 1.15 (0.60 – 2.19) | 1.11 (0.38 – 3.25) | ||||
Note: Results of logistic regression analyses testing the odds of dehydration (USG≥1.021) in relation to each of the listed covariates included together in the same model. There were significant joint effects of HWISE score, HFIAS score, and their interaction term among Daasanach women (OR: 1.35, 95% CI: 1.02–1.80, P=0.037).
Based on BMI-for-age z-scores.
p<0.001
p<0.01
p<0.05
Figure 2.
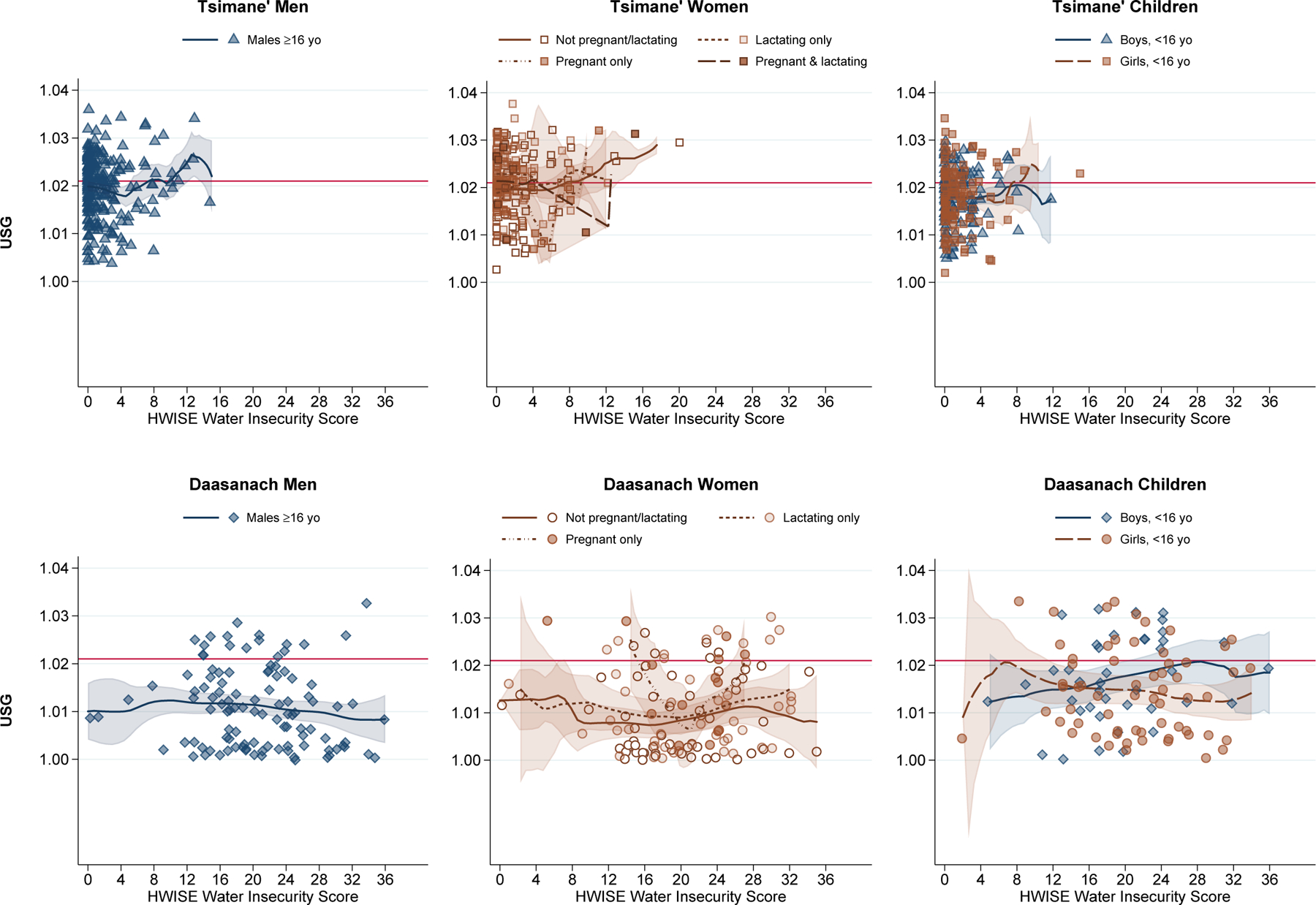
Urine Specific Gravity by HWISE Water Insecurity Score
Note: Local polynomial smooth plots with CIs
Figure 3.
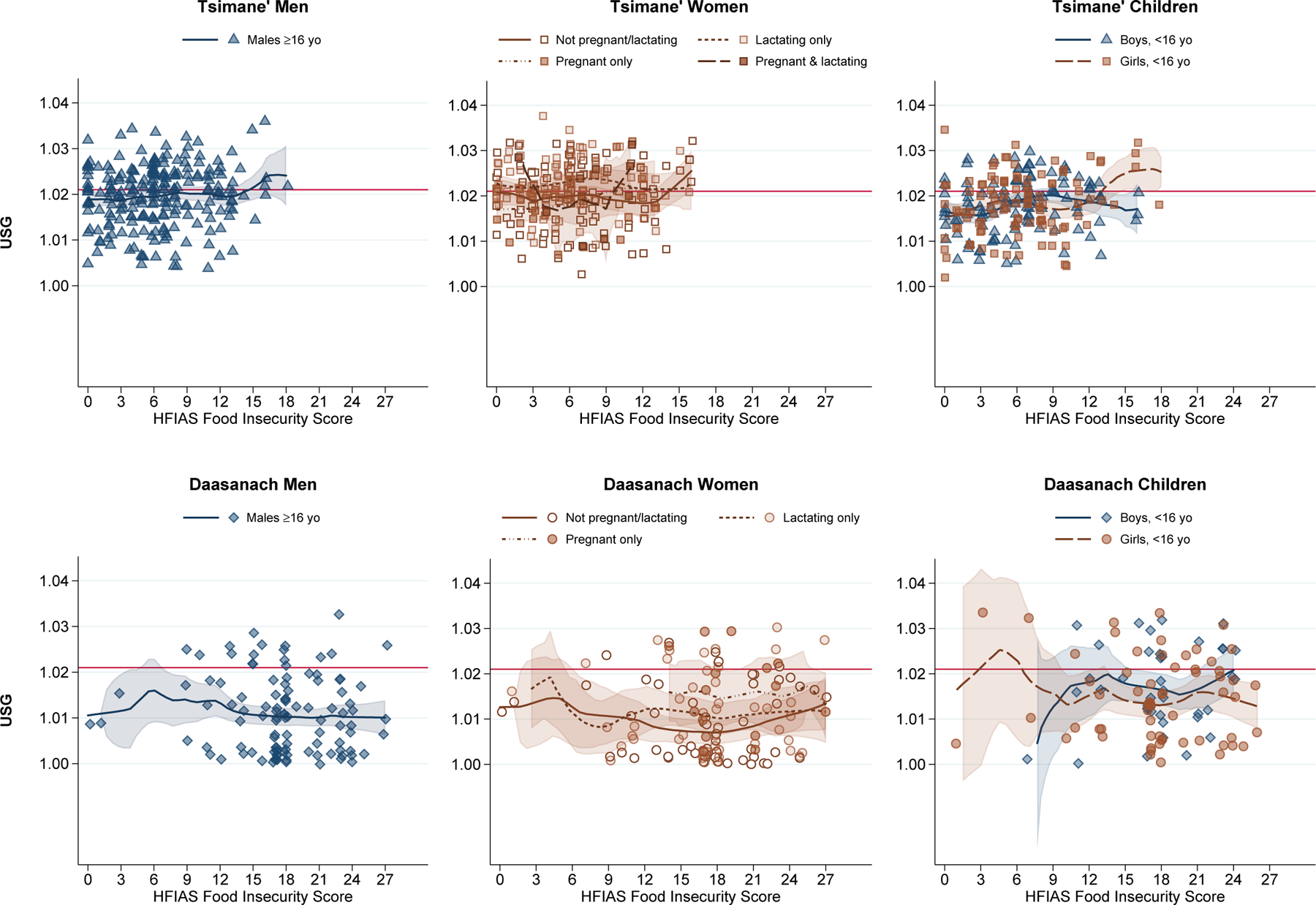
Urine Specific Gravity by HFIAS Food Insecurity Score
Note: Local polynomial smooth plots with CIs
Of the covariates in our model, heat index stood out as a significant predictor of concentrated urine. Each 2-degree Celsius increase in heat index was associated with a 23% (95% CI: 1.09–1.40, P=0.001), 34% (95% CI: 1.18–1.53, P<0.0005), and 23% (95% CI: 1.04–1.44, P=0.01) increase in the odds of concentrated urine among Tsimane’ men, women, and children respectively (Table 3; Figure 4). This relationship remained stable whether or not we included a covariate for time of day (Supplemental Tables 1–3) and appeared to be driven by ambient temperature rather than humidity (Supplemental Figures 1 and 2).
Figure 4.

Urine Specific Gravity by Heat Index
Note: Local polynomial smooth plots with CIs
Each 2-degree Celsius increase in heat index was also associated with a 48% increase in the odds of USG≥1.021 for Daasanach women (95% CI: 1.02–2.15, P=0.04) (Table 3); no such trend was observed among Daasanach men. In fact, the chi-squared test of our model fit suggested that our model for Daasanach men was no better than the null (Supplemental Table 4). Heat index was likewise not associated with hydration status among Daasanach children. However, collecting the spot urine sample in the afternoon was associated with 9.4 times the odds of having USG≥1.021 (95% CI: 2.68–32.98, P<0.0005) (Table 3; Figure 5).
Figure 5.
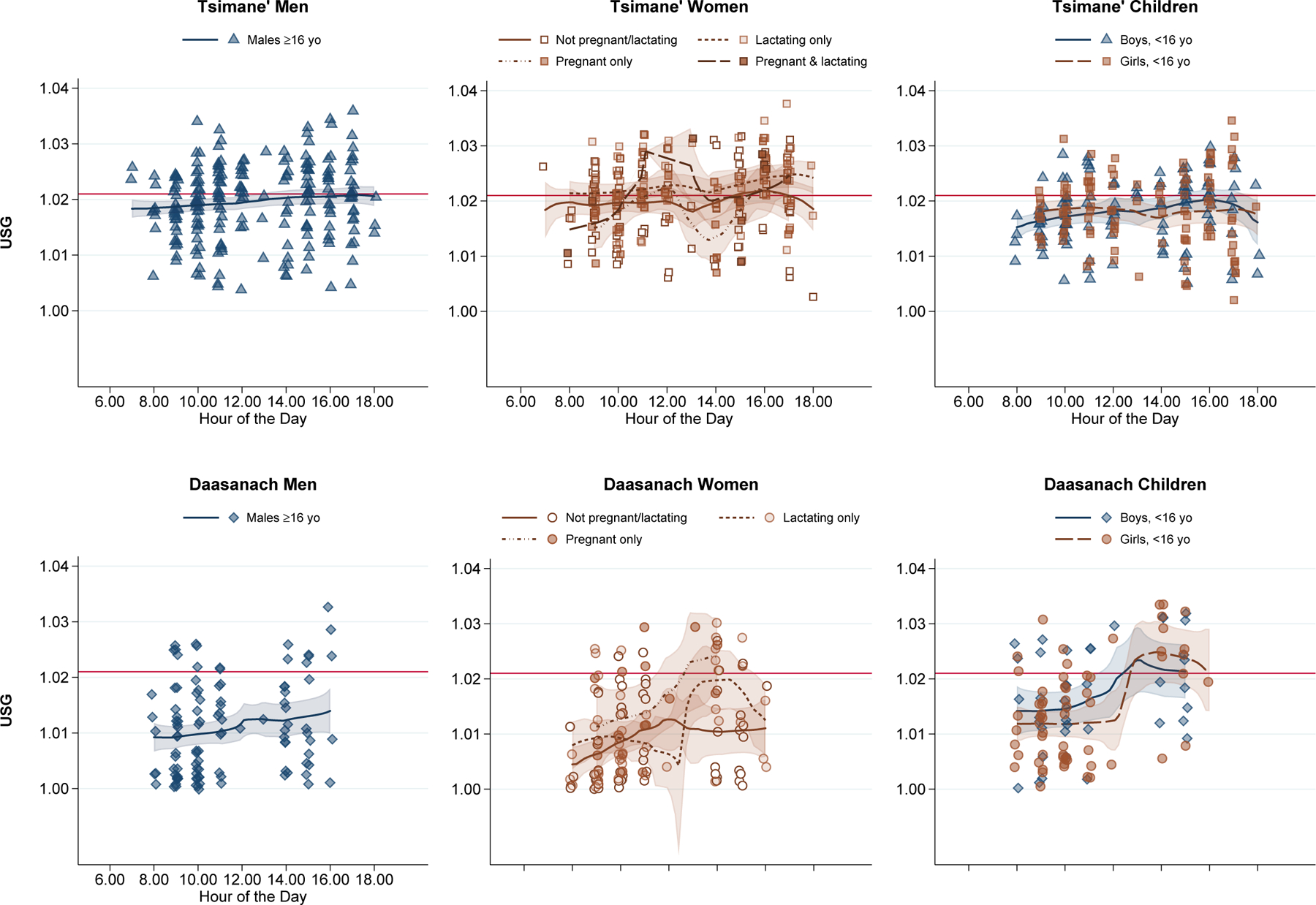
Urine Specific Gravity by Hour of the Day
Note: Local polynomial smooth plots with CIs
Lactation status was a strong predictor of hypohydration among Tsimane’ women, more than tripling the odds of having concentrated urine (OR=3.43, 95% CI: 1.64–7.16, P=0.001). Neither lactation nor pregnancy were associated with concentrated urine among Daasanach women.
While BMI was not consistently associated with USG across subgroups, BMI was associated with odds of USG≥1.021 among Daasanach women (OR: 1.32, 95% CI: 1.10–1.59, P=0.003). However, it appears that this association was driven by the women who were lactating and pregnant (Figure 6).
Figure 6.
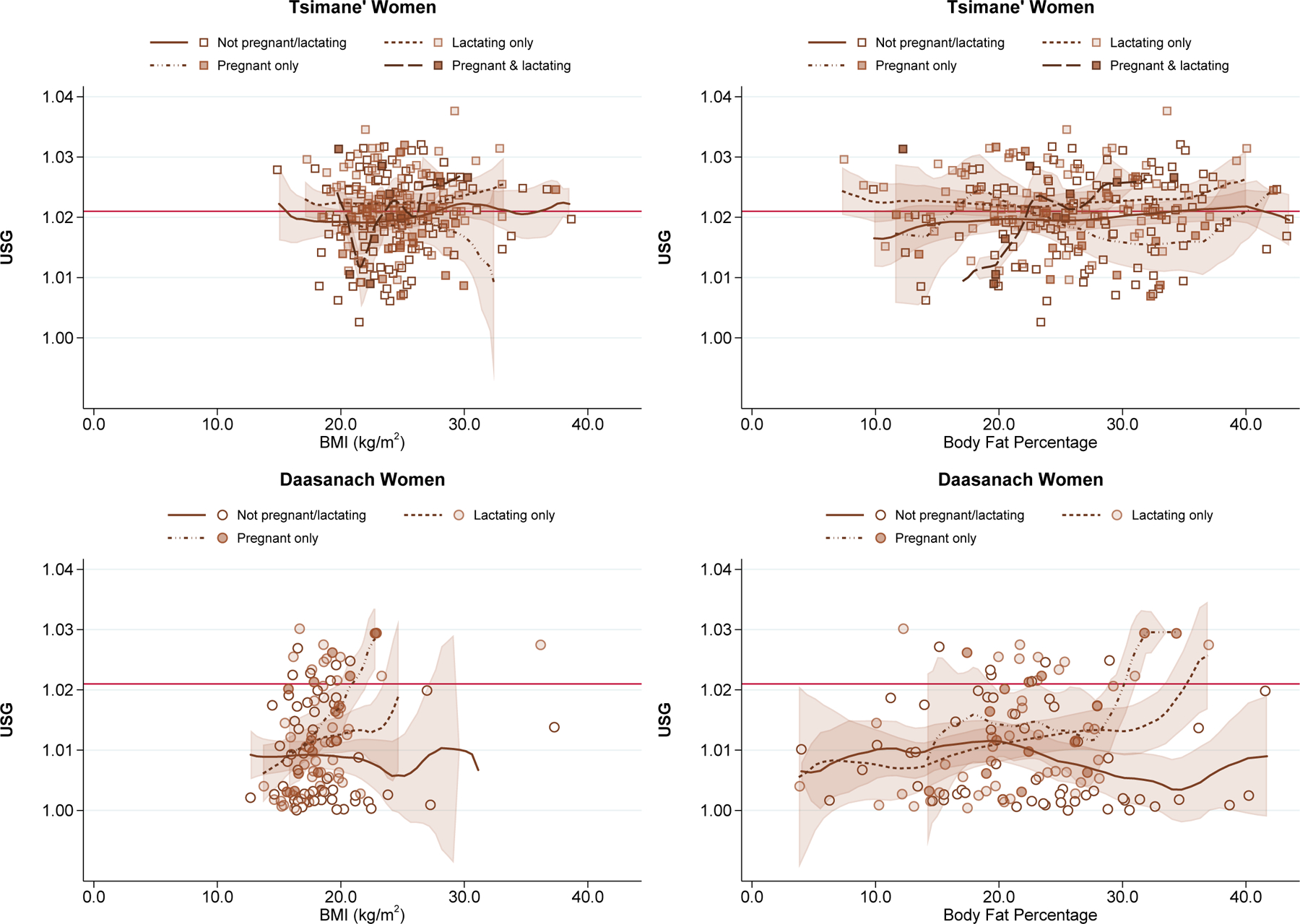
Urine Specific Gravity by Body Mass Index and Body Fat Percentage among Women Classified by Reproductive Status
Note: Local polynomial smooth plots with CIs
Figure for body fat percentage among Tsimane’ women is excluding 2 outliers with values >55%.
Within each subsample, age was only a significant predictor of concentrated urine among Tsimane’ women (OR: 1.03, 95% CI: 1.00–1.05, P =0.02).
The relationships between concentrated urine and heat index or lactation status remained consistent when adding indicators of season of birth or recent diarrhea or fever or including number of days having consumed meat or fish in the previous week (Supplemental Tables 1–6). There was no indication that either body fat percentage or calculated FFM were associated with odds of concentrated urine, with the exception of a positive relationship between body fat percentage and odds of elevated USG in Daasanach women (Supplemental Table 2). As with BMI, however, this association appeared to be driven in large part by lactating women (Figure 6).
Third aim: Hydration status in relation to recent intake of non-water beverages
With regard to the models testing associations between USG and consumption of different beverages as potential cultural buffers of heat stress and water insecurity, there were no significant associations between consumption of any of the local beverages with added sugar and odds of USG≥1.021 (Table 4). Daily milk consumption among Daasanach was not significantly associated with odds of concentrated urine, but there was a trend toward a positive relationship (Figure 7). There were no trends in tea or coffee consumption in relation to USG concentration. Having consumed any fermented chicha in the previous week appeared to be protective for Tsimane’ men (OR: 0.45, 95% CI: 0.21–0.96, P =0.04), though this was not apparent if consumption of both fermented and unfermented chicha were combined (Figure 8). On the other hand, consuming any high-alcohol content beverages in the previous week was the only significant predictor of concentrated urine among Daasanach men in our models (OR: 4.28, 95% CI: 1.12–16.25, P =0.03).
Table 4.
Multiple logistic regression analyses: odds of concentrated urine (USG≥1.021) in relation to reported non-water beverage consumption in previous week.
| Tsimane' |
Daasanach |
|||||
|---|---|---|---|---|---|---|
| Men (n=224) |
Women (n=235) |
Children (n=219)† |
Men (n=108) |
Women (n=120) |
Children (n=102)† |
|
| OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
| Any sugar-sweetened beverages | 1.48 (0.66 – 3.34) | 0.95 (0.40 – 2.23) | 1.89 (0.85 – 4.23) | 1.44 (0.41 – 5.03) | 2.66 (0.68 – 10.32) | 0.78 (0.30 – 2.01) |
| Tea or coffee, times/day | 1.42 (0.72 – 2.79) | 0.91 (0.51 – 1.61) | 1.22 (0.75 – 1.98) | |||
| Daily milk | 2.09 (0.75 – 5.83) | 1.90 (0.58 – 6.17) | 1.24 (0.40 – 3.82) | |||
| Any unfermented chicha | 1.02 (0.57 – 1.83) | 0.72 (0.40 – 1.30) | 0.58 (0.31 – 1.09) | |||
| Any homemade fermented beverages‡ | 0.45* (0.21 – 0.96) | 0.57 (0.11 – 2.89) | 2.71 (0.89 – 8.28) | 1.57 (0.32 – 7.71) | ||
| Any liquor§ | 0.59 (0.29 – 1.20) | 4.28* (1.13 – 16.25) | 4.47 (0.74 – 27.08) | |||
| Any alcohol | 0.46* (0.24 – 0.91) | 0.76 (0.17 – 3.34) | 1.89 (0.61 – 5.83) | 1.66 (0.38 – 7.35) | ||
Note: Results of logistic regression analyses adjusted for age, BMI (or BMI-for-age z-scores for children), time of day of urine collection (morning or afternoon), calculated heat index, time needed to fetch water, and (for women) reproductive status.
Further adjusted for biological sex.
Missing data for one Tsimane’ female and one Daasanach male.
Missing data for two Tsimane’ males and one Daasanach male.
p<0.001
p<0.01
p<0.05
Figure 7.
Urine Specific Gravity by Daily Milk Consumption, Daasanach
Figure 8.
Urine Specific Gravity by Any Chicha Consumption, Tsimane’
DISCUSSION
This study sought to 1) compare the prevalence of elevated urine concentration on a randomly sampled day and time across two small-scale populations living in ecologically distinct but relatively extreme thermal environments and exposed to different degrees of water and food insecurity, 2) test the association between water and food insecurity and the odds of hypohydration, and 3) test the association between local beverage consumption patterns in the previous week and the odds of hypohydration on a given day. Contrary to our hypotheses, we observed significantly higher average spot USG and prevalence of hypohydration among Tsimane’ men and women living in a water-rich, hot-humid environment compared with Daasanach living in a water-scarce, hot-arid environment. Average spot USG and prevalence of concentrated urine among children, on the other hand, were comparable across the two populations. These findings were unexpected given the higher availability of both water and water-rich foods, like fruit, in lowland Bolivia in contrast to the northern region of Kenya where there is little vegetation and fresh water is extremely scarce. Additionally, in contrast to our predictions, measures of household water insecurity and food insecurity were not independently associated with differential odds of USG ≥1.021 within either population. However, we did observe evidence of joint effects of water and food insecurity on odds of hypohydration among Tsimane’ children (when not testing for an interaction) and Daasanach women (when testing for an interaction). Finally, any consumption of fermented chicha in the previous week appeared somewhat protective against hypohydration on a given day among Tsimane’ men, while any intake of liquor in the previous week was associated with higher odds of hypohydration among Daasanach men. Otherwise, hydration status among the other subgroups was unrelated to reports of any consumption of non-water beverages in the previous week.
Of the covariates included in our models, heat index was most consistently associated with higher odds of concentrated urine, especially in the Tsimane’ subsamples. Another noteworthy finding from our data was the substantially elevated odds of concentrated urine among lactating Tsimane’ females; surprisingly, this trend was not observed among Daasanach females.
A number of factors may help explain the higher prevalence of concentrated spot urine samples among Tsimane’ adults relative to Daasanach adults. The first, most obvious factor relates to the large differences in humidity in lowland Bolivia compared with northern Kenya. Increased humidity restricts evaporation of sweat, hindering the body’s efforts to cool itself and causing inefficient or “wasteful” sweat production (Best et al., 2019; Mitchell et al., 1976). Lower sweat rates of children relative to adults (Falk et al., 1992; Meyer et al., 1992; Rivera-Brown et al., 1999) may be one reason why the prevalence of concentrated urine was not notably different between Tsimane’ and Daasanach children. Within populations, however, it appeared that heat and time of day were driving increases in urine concentration rather than differences in humidity. This was illustrated when we plotted USG against heat and humidity separately (Supplemental Figures 1 and 2), demonstrating that USG tended to go down as humidity went up. This is likely because humidity tended to be higher earlier in the morning when ambient temperatures were lower, but humidity decreased as temperatures increased throughout the day.
Body composition could be another factor contributing to differences in urinary concentration across the two populations. In the US, higher BMI has been associated with higher urine osmolality (Rosinger et al., 2016) and higher plasma tonicity (Stookey et al., 2006), on average, even among those reporting adequate water intake (Rosinger et al., 2016). This suggests that water needs may be greater for individuals with higher body mass. Tsimane’ men and women tended to have a higher BMI compared with Daasanach adults. Higher body mass relative to body surface area may also impact thermoregulatory responses and sweat rates (Davis et al., 2016), as greater surface area allows for better heat dissipation.
A third factor to explain the unexpectedly low prevalence of concentrated urine among Daasanach relative to Tsimane’ could be differences in the local disease ecology that might influence general kidney health in the two populations. For example, there is a high burden of malaria in Kenya, and some evidence suggests that malaria may in some cases cause acute kidney injury, which could increase risk of chronic kidney disease (Conroy et al., 2019; Koopmans et al., 2015). Such complications could reduce the kidneys’ ability to concentrate urine and contribute to the lower USG averages (and even some cases of hyperdilute urine) in the Daasanach sample population. Any impacts of kidney injury on the development of chronic kidney disease is likely to be more advanced among adults, so this could be another reason for the difference in urine concentration among adults but not children across the two populations.
Overall, our study findings suggest that USG is so dependent on physiological and weather-related factors among populations living in extreme thermal environments without access to climate control that measures of chronic water insecurity may not strongly predict differences in hydration status. Our discovery that water insecurity was not independently related to urine concentration contrast with a previous report of substantially increased odds of concentrated urine among children (though not adults) in relation to increased water insecurity (Rosinger, 2018). However, water insecurity for that study was measured using a different instrument, and those data were collected after a flood that displaced many households, destroyed crops, and led to an elevated prevalence of diarrheal infections. To our knowledge, there are no other studies that have examined the relationship between water or food insecurity and hydration status and, thus, no other studies to which we can compare our findings. Perhaps there was too little variability in water insecurity scores among Tsimane’ and too low a prevalence of concentrated urine among Daasanach to detect an independent association between these variables when controlling for covariates. Additionally, the HWISE water insecurity scale used for this study is a measurement of perceived water stress over the previous four weeks, whereas USG is an acute measure of hydration status in the moment and can vary by the hour. The distinct time scales may also reduce the strength of relationship between these variables. Nonetheless, it is notable that the point estimates for water insecurity were all positive, with odds ratios ranging from 1.05 to 1.28 for just a 1-point difference in water insecurity score. It is also intriguing that water insecurity combined with food insecurity was associated with increased odds of hypohydration among Tsimane’ children and Daasanach women, though it is unclear why similar trends were not observed in the other subsamples. Previous research has also found joint effects of water and food insecurity on other health outcomes. For example, in the Galapagos, households that lacked access to clean water and experienced food insecurity had higher odds of the dual burden of overweight/chronic diseases and undernutrition/infectious diseases (Thompson et al.).
Higher USG in relation to lactation status has previously been observed among Tsimane’ women (Rosinger, 2015) and is not surprising given the approximately 700–800 mL/day of milk produced by lactating women (McKenzie et al., 2017; Neville et al., 1988), which will translate into greater water loss. Limited research has suggested that at low levels of water intake, lactating women tended to have higher USG than non-lactating women (McKenzie et al., 2017). This was not the same for pregnant women, suggesting that low water intake may create a greater strain among lactating compared with pregnant women. Hence, if water intake does not increase to compensate for the greater water loss from lactation, particularly among women already losing substantial body water from sweating in humid environments, increased urine concentration is a likely consequence. The resulting dehydration could in turn influence milk composition, though the data in this area are limited (Rosinger, 2020). Further research on this topic is warranted, as is research to understand what physiological, environmental, and behavioral factors might contribute to our observations of a significant association between lactation status among Tsimane’ women but not among Daasanach women.
Apart from the potential effects of dehydration on the health and well-being of pregnant and lactating mothers, dehydration could also impact that long-term health of offspring who were exposed to dehydration in utero. Animal studies, for example, have suggested that water deprivation during pregnancy could negatively impact birth weight (Ross & Desai, 2005), renal morphology and function (Svitok et al., 2019), and the renin–angiotensin system (Guan et al., 2009; Zhang et al., 2011) of offspring, potentially influencing their long-term risk for hypertension and cardiovascular disease. There is likewise reason to hypothesize that prenatal exposures to water deficits could alter offspring’s own ability to regulate water balance (Rosinger, 2020), which is why we tested for an association with birth season. Though we found no association between birth season and odds of hypohydration, future work with higher resolution data on prenatal exposures to dehydration might help us better understand how variation within and across populations in thirst, consumption of liquids, and vulnerability or resilience to dehydration and heat stress affect differential risk for hypohydration later in life.
Our analyses of the relationship between recent non-water beverage consumption and urine concentration suggested that, with the exception of fermented chicha consumption among Tsimane’, consumption of other non-water beverages in the previous week did not provide a notable hydration advantage. The finding that any consumption of fermented chicha was associated with lower odds of concentrated urine among Tsimane’ men is an important finding and in line with research suggesting that low alcohol beers can be hydrating when consumed in a dehydrated state (Hobson & Maughan, 2010). The fermentation process may also be beneficial for reducing bacterial concentration of water, thereby potentially providing a safer form of hydration (Rosinger & Bethancourt, 2020). High concentrations of alcohol, on the other hand, may have at least small, acute diuretic effects (Polhuis et al., 2017), and this may explain why we observed an increased odds of concentrated urine among Daasanach men who reported consuming liquor in the previous week.
It is somewhat surprising that there was not an association between USG and consumption of milk among Daasanach, which has been demonstrated in previous research to be a particularly hydrating beverage (Maughan et al., 2016; Maughan et al., 2019). Milk consumption may not have been as frequent at the time of data collection as during the rainy season, when there is more pasture for livestock and, thus, higher milk production. It may also be that greater resolution in the amounts of milk consumed, particularly on the day and morning prior to sample collection, would be needed to observe an association. Similarly, data on consumption of amounts of beverages with added sugar the day and morning prior to sample collection may be necessary to more accurately assess any relationships between recent consumption of sugar-sweetened beverages and hydration status in these populations.
While we controlled for a host of environmental, behavioral, and dietary variables and study procedures were mostly consistent across sites, other unmeasured physiological, behavioral, lifestyle, and environmental factors could also contribute to the variation in USG within and across Tsimane’ and Daasanach populations and would be valuable avenues of research to pursue in future studies. For example, without measures of sweat rates and insensible water loss or the volume of water and other beverages consumed, it is unclear to what degree the higher prevalence of hypohydration among Tsimane’ is attributable to less fluid intake versus greater volume of body water excretion. There may have been differences in amount of time spent doing moderate or vigorous labor, household chores, or other activities the day or morning prior to sample collection; physical activity was not measured in this study and could have been a source of noise in our models. We also do not know if differences existed in the amount of time spent in direct sunlight versus shade in the day and hours prior to collecting the urine sample; it would be helpful for future studies to collect information on recent and regular sun exposure.
Variation in dietary composition within and across the two populations may also influence measures of urine concentration. USG can be elevated not only as a result of reduced water input and excretion but also as an increase in excreted solutes, which could occur as a results of protein breakdown (Cheuvront & Kenefick, 2014). Protein intake has been associated with higher urine osmolality in a US study sample (Yeh et al., 2015), and we suspect that protein intake might be higher, on average, among Tsimane’. We found no effect of controlling for days of meat, poultry, or fish consumption as a proxy of solute load. However, higher resolution data on frequency and amount of all protein-rich foods consumed, as well as sodium intake, prior to measuring hydration status may help to better assess whether distinct dietary habits contribute to differences in USG across the two populations.
Furthermore, as previously mentioned, differences in disease ecology could play a role in different USG concentrations. Parasitic infections can be associated with elevated USG if they impact the kidneys, gastrointestinal tract, and other tissues involved in maintaining water homeostasis (Rosinger, 2020; Rosinger et al., 2018). We did not observe an association between self-reports of recent diarrhea or fever and USG, but lack of diarrhea or fever does not necessarily rule out the possibility of an active parasitic infection. Future research investigating the role of disease history and both chronic and acute immunological stressors on risk of hypohydration would be valuable.
An additional limitation of this study is the use of spot urine samples, which can lead to measurements of USG that are more variable than first morning void or 24-hour urine samples (Gowans & Fraser, 1987). Notwithstanding, data from spot urine have important public health implications in light of recent epidemiological research suggesting that repeated bouts of dehydration and heat stress may be responsible for the troublingly high prevalence and incidence of chronic kidney disease among agricultural workers living in hot climates (Correa-Rotter et al., 2014; García-Trabanino et al., 2015; Glaser et al., 2016; Laws et al., 2015; Mix et al., 2018). Experiments with rodents have further supported the hypothesis the recurrent heat-induced dehydration may be associated with renal injury and the progression of kidney disease (García-Arroyo et al., 2016; Hilliard et al., 2016; Roncal Jimenez et al., 2014). This suggests that even evidence of hypohydration in a single spot urine sample could provide an indicator of increased risk for kidney injury if we infer that the concentrated urine in one random sample may be predictive of recurring hypohydration in repeat random samples.
Together the findings from this study contribute to our understanding of how physiological, climate, and behavioral factors might interact differently across distinct ecological contexts to shape risk of hypohydration and, consequently, long-term kidney health. To date, much of the research investigating thermoregulation and hydration in relation to heat and humidity have been in relation to exercise performance among elite athletes or during intense agricultural labor. This study provides a distinct perspective on hydration status among two small-scale populations that are not only active by necessity but also have limited means by which to carry and consume water throughout the day and scarce access to clean water. Moreover, the majority of research has been conducted among young men or women and has excluded pregnant and lactating women. A major strength of this study is the inclusion of children, men, and women, including pregnant and lactating women, allowing for the examination of physiological responses to extreme environments at different stages of life history. With the forecasted droughts in much of Africa (Ahmadalipour et al., 2019) and projected increases in global temperatures (Burek et al., 2016; Kjellstrom et al., 2016; Mekonnen & Hoekstra, 2016; Xu et al., 2020), these issues remain a concern for these populations and others living in comparably harsh environmental and water insecure conditions. Distinct combinations of physiological, cultural, ecological, environmental, and political factors may influence how a given population experiences water insecurity. Therefore, further research among diverse populations in distinct ecological settings is needed to gain a more comprehensive perspective on how water and food insecurity, environmental landscape, weather, physiology, and behavior interact to influence risk of dehydration and corresponding health issues.
Conclusion
In summary, this study did not find evidence to support our hypotheses that the prevalence and odds of hypohydration would be higher in a more water insecure region and in relation to greater degrees of recent household water or food insecurity, though there was limited evidence for potential joint effects of water and food insecurity on hydration status. Instead, heat index and lactation status stood out as the strongest predictors of elevated urine concentration within Tsimane’ and Daasanach sample populations. This study highlights numerous avenues to pursue in future research on inter- and intra-population differences in risk of hypohydration. Specifically, it calls for further investigations into the interactions between environmental, physiological, reproductive, immunological, behavioral, and dietary factors that may modify the degree to which individuals or populations are more vulnerable to or resilient against becoming dehydrated and developing associated health issues. It is particularly important to pursue further research on these factors in the context of regular exposure to relatively high thermal stress combined with water and food insecurity. Continued work of this nature, beyond laboratory settings and among underserved populations, is critical given the projected increases in global temperatures, drought, and flooding, all which will further augment current water and food insecurity concerns around the world.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the Gran Consejo Tsimane’, the Kenya Medical Research Institute, the National Museums of Kenya, the Ileret Health Clinic, our translators (Manuel Roca Moye, Elias Hiza Nate, Robin Nate Roca, Luke Lomeiku, Samuel Esho, and Joshua Koribok), research assistants (Jessica Saunders, Shiva Dhanasekar, Celine LaTona, Alysha Kelyman, Jason John), community leaders from each participating community, and all study participants.
FUNDING STATEMENT
This work was funded by the National Science Foundation (NSF ARCH #1624398; NSF REU #1930719; NSF CNH2-S #1924322) and a Pennsylvania State University Social Science Research Institute (SSRI) Human Health and Environment Seed Grant and funds from the College of Health and Human Development.
Footnotes
CONFLICTS OF INTEREST
HJB has consulted for Novo Nordisk on a project unrelated to this one. There are no other conflicts of interest to declare.
Publisher's Disclaimer: This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ajhb.23447
REFERENCES
- Ahmadalipour A, Moradkhani H, Castelletti A, & Magliocca N (2019). Future drought risk in Africa: Integrating vulnerability, climate change, and population growth. Science of The Total Environment, 662, 672–686. doi: 10.1016/j.scitotenv.2019.01.278 [DOI] [PubMed] [Google Scholar]
- Anderson GB, Bell ML, & Peng RD (2013). Methods to calculate the heat index as an exposure metric in environmental health research. Environmental Health Perspectives, 121(10), 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong LE (2007). Assessing Hydration Status: The Elusive Gold Standard. Journal of the American College of Nutrition, 26(sup5), 575S–584S. doi: 10.1080/07315724.2007.10719661 [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Pumerantz AC, Fiala KA, Roti MW, Kavouras SA, Casa DJ, & Maresh CM (2010). Human hydration indices: acute and longitudinal reference values. Int J Sport Nutr Exerc Metab, 20(2), 145–153. [DOI] [PubMed] [Google Scholar]
- Bain R, Cronk R, Hossain R, Bonjour S, Onda K, Wright J, … Bartram J (2014). Global assessment of exposure to faecal contamination through drinking water based on a systematic review. Tropical Medicine & International Health, 19(8), 917–927. doi: 10.1111/tmi.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best A, Lieberman DE, & Kamilar JM (2019). Diversity and evolution of human eccrine sweat gland density. Journal of Thermal Biology, 84, 331–338. doi: 10.1016/j.jtherbio.2019.07.024 [DOI] [PubMed] [Google Scholar]
- Bethancourt HJ, Leonard WR, Tanner S, Schultz AF, & Rosinger AY (2019). Longitudinal Changes in Measures of Body Fat and Diet Among Adult Tsimane’ Forager-Horticulturalists of Bolivia, 2002–2010. Obesity, 27(8), 1347–1359. doi: 10.1002/oby.22556 [DOI] [PubMed] [Google Scholar]
- Brewis A, Workman C, Wutich A, Jepson W, & Young S (2019). Household water insecurity is strongly associated with food insecurity: Evidence from 27 sites in low- and middle-income countries. Am J Hum Biol, e23309. doi: 10.1002/ajhb.23309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek P, Satoh Y, Fischer G, Kahil M, Scherzer A, Tramberend S, … Flörke M (2016). Water futures and solution: fast track initiative (final report). IIASA, Laxenburg, Austria. [Google Scholar]
- Chaplin-Kramer R, Sharp RP, Weil C, Bennett EM, Pascual U, Arkema KK, … Daily G (2019). Global modeling of nature’s contributions to people. Science, 366(6462), 255–258. doi: 10.1126/science.aaw3372 [DOI] [PubMed] [Google Scholar]
- Cheuvront SN, Ely BR, Kenefick RW, & Sawka MN (2010). Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr, 92(3), 565–573. doi: 10.3945/ajcn.2010.29490 [DOI] [PubMed] [Google Scholar]
- Cheuvront SN, & Kenefick RW (2014). Dehydration: Physiology, Assessment, and Performance Effects. Comprehensive Physiology, 4(1), 257–285. doi: 10.1002/cphy.c130017 [DOI] [PubMed] [Google Scholar]
- Cheuvront SN, Muñoz CX, & Kenefick RW (2016). The void in using urine concentration to assess population fluid intake adequacy or hydration status. The American Journal of Clinical Nutrition, 104(3), 553–556. doi: 10.3945/ajcn.115.129858 [DOI] [PubMed] [Google Scholar]
- Coates J, Swindale A, & Bilinsky P (2007). Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3). Washington, D.C.: Food and Nutrition Technical Assistance Project, Academy for Educational Development. [Google Scholar]
- Conroy AL, Opoka RO, Bangirana P, Idro R, Ssenkusu JM, Datta D, … John CC (2019). Acute kidney injury is associated with impaired cognition and chronic kidney disease in a prospective cohort of children with severe malaria. BMC Med, 17(1), 98. doi: 10.1186/s12916-019-1332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Rotter R, Wesseling C, & Johnson RJ (2014). CKD of Unknown Origin in Central America: The Case for a Mesoamerican Nephropathy. American Journal of Kidney Diseases, 63(3), 506–520. doi: 10.1053/j.ajkd.2013.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JK, Baker LB, Barnes K, Ungaro C, & Stofan J (2016). Thermoregulation, Fluid Balance, and Sweat Losses in American Football Players. Sports Medicine, 46(10), 1391–1405. doi: 10.1007/s40279-016-0527-8 [DOI] [PubMed] [Google Scholar]
- European Food Safety Association. (2010). EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA); Scientific Opinion on Dietary reference values for water. EFSA Journal, 8(3), 1459. [Google Scholar]
- Falk B, Bar-Or O, & MacDougall JD (1992). Thermoregulatory responses of pre-, mid-, and late-pubertal boys to exercise in dry heat. Medicine and science in sports and exercise, 24(6), 688–694. [PubMed] [Google Scholar]
- García-Arroyo FE, Cristóbal M, Arellano-Buendía AS, Osorio H, Tapia E, Soto V, … Sánchez-Lozada L-G (2016). Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 311(1), R57–R65. doi: 10.1152/ajpregu.00354.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Trabanino R, Jarquín E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, … Barregard L (2015). Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador – A cross-shift study of workers at risk of Mesoamerican nephropathy. Environmental Research, 142, 746–755. doi: 10.1016/j.envres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Gebre Y (2012). Environmental Change, Food Crises and Violence in Dassanech, Southern Ethiopia. Berlin, Germany: Freie Universität Berlin, Research Unit Peace and Conflict Studies. [Google Scholar]
- Glaser J, Lemery J, Rajagopalan B, Diaz HF, García-Trabanino R, Taduri G, … Johnson RJ (2016). Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clinical Journal of the American Society of Nephrology, 11(8), 1472–1483. doi: 10.2215/cjn.13841215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans EM, & Fraser CG (1987). Despite correlation, random spot and 24-h urine specimens are not interchangeable. Clin Chem, 33(6), 1080–1081. [PubMed] [Google Scholar]
- Guan J, Mao C, Xu F, Geng C, Zhu L, Wang A, & Xu Z (2009). Prenatal dehydration alters renin–angiotensin system associated with angiotensin-increased blood pressure in young offspring. Hypertension Research, 32(12), 1104–1111. doi: 10.1038/hr.2009.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Trumble B, Blackwell AD, Beheim B, Davis H, … Kaplan H (2017). The Tsimane Health and Life History Project: Integrating anthropology and biomedicine. Evolutionary Anthropology: Issues, News, and Reviews, 26(2), 54–73. doi:doi: 10.1002/evan.21515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna EG, & Tait PW (2015). Limitations to Thermoregulation and Acclimatization Challenge Human Adaptation to Global Warming. International journal of environmental research and public health, 12(7), 8034–8074. doi: 10.3390/ijerph120708034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard LM, Colafella KMM, Bulmer LL, Puelles VG, Singh RR, Ow CPC, … Denton KM (2016). Chronic recurrent dehydration associated with periodic water intake exacerbates hypertension and promotes renal damage in male spontaneously hypertensive rats. Scientific Reports, 6(1), 33855. doi: 10.1038/srep33855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RM, & Maughan RJ (2010). Hydration Status and the Diuretic Action of a Small Dose of Alcohol. Alcohol and Alcoholism, 45(4), 366–373. doi: 10.1093/alcalc/agq029 [DOI] [PubMed] [Google Scholar]
- Hooper L, Bunn D, Jimoh FO, & Fairweather-Tait SJ (2014). Water-loss dehydration and aging. Mechanisms of Ageing and Development, 136–137, 50–58. doi: 10.1016/j.mad.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2005). Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: The National Academies Press. [Google Scholar]
- Johnson RJ, Rodriguez-Iturbe B, Roncal-Jimenez C, Lanaspa MA, Ishimoto T, Nakagawa T, … Sanchez-Lozada LG (2014). Hyperosmolarity drives hypertension and CKD—water and salt revisited. Nature Reviews Nephrology, 10(7), 415–420. doi: 10.1038/nrneph.2014.76 [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Sánchez-Lozada LG, Newman LS, Lanaspa MA, Diaz HF, Lemery J, … Roncal-Jimenez CA (2019). Climate Change and the Kidney. Annals of Nutrition and Metabolism, 74(suppl 3)(3), 38–44. doi: 10.1159/000500344 [DOI] [PubMed] [Google Scholar]
- Kenney WL, & Buskirk ER (1995). Functional consequences of sarcopenia: effects on thermoregulation. J Gerontol A Biol Sci Med Sci, 50 Spec No, 78–85. doi: 10.1093/gerona/50a.special_issue.78 [DOI] [PubMed] [Google Scholar]
- Kjellstrom T, Briggs D, Freyberg C, Lemke B, Otto M, & Hyatt O (2016). Heat, Human Performance, and Occupational Health: A Key Issue for the Assessment of Global Climate Change Impacts. Annual Review of Public Health, 37(1), 97–112. doi: 10.1146/annurev-publhealth-032315-021740 [DOI] [PubMed] [Google Scholar]
- Koopmans LC, van Wolfswinkel ME, Hesselink DA, Hoorn EJ, Koelewijn R, van Hellemond JJ, & van Genderen PJ (2015). Acute kidney injury in imported Plasmodium falciparum malaria. Malar J, 14, 523. doi: 10.1186/s12936-015-1057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft TS, Stieglitz J, Trumble BC, Martin M, Kaplan H, & Gurven M (2018). Nutrition transition in 2 lowland Bolivian subsistence populations. American Journal of Clinical Nutrition, 1183–1195. doi: 10.1093/ajcn/nqy250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, … Zhong M (2018). The Lancet Commission on pollution and health. The Lancet, 391(10119), 462–512. doi: 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, … McClean MD (2015). Changes in kidney function among Nicaraguan sugarcane workers. International Journal of Occupational and Environmental Health, 21(3), 241–250. doi: 10.1179/2049396714Y.0000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WF, Cerundolo LH, Pikosky MA, Gaine PC, Maresh CM, Armstrong LE, … Rodriguez NR (2006). Effects of Dietary Protein Intake on Indexes of Hydration. Journal of the American Dietetic Association, 106(4), 587–589. doi: 10.1016/j.jada.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Maughan RJ, Watson P, Cordery PA, Walsh NP, Oliver SJ, Dolci A, … Galloway SD (2016). A randomized trial to assess the potential of different beverages to affect hydration status: development of a beverage hydration index. The American Journal of Clinical Nutrition, 103(3), 717–723. doi: 10.3945/ajcn.115.114769 [DOI] [PubMed] [Google Scholar]
- Maughan RJ, Watson P, Cordery PAA, Walsh NP, Oliver SJ, Dolci A, … Galloway SDR (2019). Sucrose and Sodium but not Caffeine Content Influence the Retention of Beverages in Humans Under Euhydrated Conditions. 29(1), 51. doi: 10.1123/ijsnem.2018-0047 [DOI] [PubMed] [Google Scholar]
- McKenzie AL, Perrier ET, Guelinckx I, Kavouras SA, Aerni G, Lee EC, … Armstrong LE (2017). Relationships between hydration biomarkers and total fluid intake in pregnant and lactating women. European Journal of Nutrition, 56(6), 2161–2170. doi: 10.1007/s00394-016-1256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen MM, & Hoekstra AY (2016). Four billion people facing severe water scarcity. Science Advances, 2(2), e1500323. doi: 10.1126/sciadv.1500323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Bar-Or O, MacDougall D, & Heigenhauser GJ (1992). Sweat electrolyte loss during exercise in the heat: effects of gender and maturation. Medicine and science in sports and exercise, 24(7), 776–781. [PubMed] [Google Scholar]
- Mitchell D, Senay LC, Wyndham CH, Rensburg A. J. v., Rogers GG, & Strydom NB (1976). Acclimatization in a hot, humid environment: energy exchange, body temperature, and sweating. Journal of Applied Physiology, 40(5), 768–778. doi: 10.1152/jappl.1976.40.5.768 [DOI] [PubMed] [Google Scholar]
- Mix J, Elon L, Vi Thien Mac V, Flocks J, Economos E, Tovar-Aguilar AJ, … McCauley LA (2018). Hydration status, kidney function, and kidney injury in Florida agricultural workers. Journal of occupational and environmental medicine, 60(5), e253–e260. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Latzka WA, & Sawka MN (1995). Control of thermoregulatory sweating is altered by hydration level and exercise intensity. Journal of Applied Physiology, 79(5), 1434–1439. doi: 10.1152/jappl.1995.79.5.1434 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. (1998). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults - the evidence report. National Heart, Lung, and Blood Institute. Bethesda, MD: Retrieved from https://www.nhlbi.nih.gov/health-pro/guidelines/archive/clinical-guidelines-obesity-adults-evidence-report. [PubMed] [Google Scholar]
- Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, … Archer P (1988). Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. The American Journal of Clinical Nutrition, 48(6), 1375–1386. doi: 10.1093/ajcn/48.6.1375 [DOI] [PubMed] [Google Scholar]
- NOAA National Weather Service Weather Prediction Center. (2014). The Heat Index Equation. Retrieved from https://www.wpc.ncep.noaa.gov/html/heatindex_equation.shtml
- Opiyo F, Nyangito M, Wasonga OV, & Omondi P (2014). Trend Analysis of Rainfall and Temperature Variability in Arid Environment of Turkana, Kenya. Environmental Research Journal, 8(2), 30–43. [Google Scholar]
- Polhuis KCMM, Wijnen AHC, Sierksma A, Calame W, & Tieland M (2017). The Diuretic Action of Weak and Strong Alcoholic Beverages in Elderly Men: A Randomized Diet-Controlled Crossover Trial. Nutrients, 9(7), 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Brown AM, Gutiérrez R, Gutiérrez JC, Frontera WR, & Bar-Or O (1999). Drink composition, voluntary drinking, and fluid balance in exercising, trained, heat-acclimatized boys. Journal of Applied Physiology, 86(1), 78–84. doi: 10.1152/jappl.1999.86.1.78 [DOI] [PubMed] [Google Scholar]
- Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, … Johnson RJ (2014). Fructokinase activity mediates dehydration-induced renal injury. Kidney International, 86(2), 294–302. doi: 10.1038/ki.2013.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger A (2015). Dehydration among lactating mothers in the Amazon: A neglected problem. American Journal of Human Biology, 27(4), 576–578. doi: 10.1002/ajhb.22672 [DOI] [PubMed] [Google Scholar]
- Rosinger A, & Tanner S (2015). Water from fruit or the river? Examining hydration strategies and gastrointestinal illness among Tsimane’ adults in the Bolivian Amazon. Public Health Nutrition, 18(6), 1098–1108. doi: 10.1017/S1368980014002158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger AY (2018). Household water insecurity after a historic flood: Diarrhea and dehydration in the Bolivian Amazon. Social Science & Medicine, 197, 192–202. doi: 10.1016/j.socscimed.2017.12.016 [DOI] [PubMed] [Google Scholar]
- Rosinger AY (2020). Biobehavioral variation in human water needs: How adaptations, early life environments, and the life course affect body water homeostasis. American Journal of Human Biology, 32(1), e23338. doi: 10.1002/ajhb.23338 [DOI] [PubMed] [Google Scholar]
- Rosinger AY, & Bethancourt HJ (2020). Chicha as Water: Traditional Fermented Beer Consumption among Forager-Horticulturalists in the Bolivian Amazon. In Hockings K & Dunbar R (Eds.), Alcohol and Humans: A Long and Social Affair (pp. 147–162): Oxford University Press. [Google Scholar]
- Rosinger AY, Lawman HG, Akinbami LJ, & Ogden CL (2016). The role of obesity in the relation between total water intake and urine osmolality in US adults, 2009–2012. The American Journal of Clinical Nutrition, 104(6), 1554–1561. doi: 10.3945/ajcn.116.137414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger AY, Pontzer H, Raichlen DA, Wood BM, Tanner SN, & Sands JM (2019). Age-related decline in urine concentration may not be universal: Comparative study from the U.S. and two small-scale societies. American Journal of Physical Anthropology, 168(4), 705–716. doi: 10.1002/ajpa.23788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger AY, Young SL, Collins SM, Haider SR, Mishra P, Nagai HT, … Downs JA (2018). Schistosomiasis and hydration status: Schistosoma haematobium, but not Schistosoma mansoni increases urine specific gravity among rural Tanzanian women. American Journal of Physical Anthropology, 166(4), 952–959. doi: 10.1002/ajpa.23479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MG, & Desai M (2005). Gestational programming: population survival effects of drought and famine during pregnancy. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 288(1), R25–R33. doi: 10.1152/ajpregu.00418.2004 [DOI] [PubMed] [Google Scholar]
- Rothfusz LP (1990). The heat index equation. National Weather Service Technical Attachment (SR 90–23). [Google Scholar]
- Ruiz-Mallén I, Fernández-Llamazares Á, & Reyes-García V (2017). Unravelling local adaptive capacity to climate change in the Bolivian Amazon: the interlinkages between assets, conservation and markets. Climatic Change, 140(2), 227–242. doi: 10.1007/s10584-016-1831-x [DOI] [Google Scholar]
- Sawka MN, Cheuvront SN, & Carter R (2005). Human water needs. Nutr Rev, 63(suppl 1), S30–S39. doi: 10.1111/j.1753-4887.2005.tb00152.x [DOI] [PubMed] [Google Scholar]
- Sawka MN, Montain SJ, & Latzka WA (2001). Hydration effects on thermoregulation and performance in the heat. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 128(4), 679–690. doi: 10.1016/S1095-6433(01)00274-4 [DOI] [PubMed] [Google Scholar]
- Sawka MN, Young AJ, Francesconi RP, Muza SR, & Pandolf KB (1985). Thermoregulatory and blood responses during exercise at graded hypohydration levels. Journal of Applied Physiology, 59(5), 1394–1401. doi: 10.1152/jappl.1985.59.5.1394 [DOI] [PubMed] [Google Scholar]
- Serem D (2012, November 29, 2012). For villages in Turkana, Kenya, a new initiative that brings clean water to the community is life-changing. Retrieved from https://www.unicef.org/wash/kenya_66520.html
- Stookey JD, Barclay D, Arieff A, & Popkin BM (2006). The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr, 61(2), 190–199. [DOI] [PubMed] [Google Scholar]
- Stookey JD, Kavouras SA, Suh H, & Lang F (2020). Underhydration Is Associated with Obesity, Chronic Diseases, and Death Within 3 to 6 Years in the U.S. Population Aged 51–70 Years. Nutrients, 12(4), 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitok P, Okuliarova M, Varga I, & Zeman M (2019). Renal impairment induced by prenatal exposure to angiotensin II in male rat offspring. Experimental Biology and Medicine, 244(11), 923–931. doi: 10.1177/1535370219851110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AL, Nicholas KM, Watson E, Terán E, & Bentley ME Water, food, and the dual burden of disease in Galápagos, Ecuador. American Journal of Human Biology, 0(0), e23344. doi: 10.1002/ajhb.23344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF and WHO. (2017). Progress on drinking water, sanitation and hygiene: 2017 update and SDG baselines. Geneva: United Nations Children’s Fund (UNICEF) and World Health Organization (WHO). [Google Scholar]
- UNICEF and WHO. (2019). Progress on household drinking water, sanitation and hygiene 2000–2017. Special focus on inequalities. New York: United Nations Children’s Fund (UNICEF) and World Health Organization (WHO). [Google Scholar]
- World Health Organization. Growth reference 5–19 years. Application Tools. WHO AnthroPlus software. Retrieved from https://www.who.int/growthref/tools/en/
- World Health Organization. (2010). Nutrition Landscape Information System (NLIS). Country profile indicators: interpretation guide. Geneva, Switzerland: World Health Organization. [Google Scholar]
- World Health Organization. (2018). Drinking-water. Retrieved from https://www.who.int/en/news-room/fact-sheets/detail/drinking-water
- World Weather Online. Lodwar Monthly Climate Averages. Retrieved from https://www.worldweatheronline.com/lodwar-weather-averages/rift-valley/ke.aspx
- World Weather Online. San Borja Monthly Climate Averages. Retrieved from https://www.worldweatheronline.com/san-borja-weather-averages/el-beni/bo.aspx
- Xu C, Kohler TA, Lenton TM, Svenning J-C, & Scheffer M (2020). Future of the human climate niche. Proceedings of the National Academy of Sciences, 201910114. doi: 10.1073/pnas.1910114117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H-C, Lin Y-S, Kuo C-C, Weidemann D, Weaver V, Fadrowski J, … Navas-Acien A (2015). Urine osmolality in the US population: Implications for environmental biomonitoring. Environmental Research, 136, 482–490. doi: 10.1016/j.envres.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Boateng GO, Jamaluddine Z, Miller JD, Frongillo EA, Neilands TB, … Stoler J (2019). The Household Water InSecurity Experiences (HWISE) Scale: development and validation of a household water insecurity measure for low-income and middle-income countries. BMJ Global Health, 4(5), e001750. doi: 10.1136/bmjgh-2019-001750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Collins SM, Boateng GO, Neilands TB, Jamaluddine Z, Miller JD, … Wutich A (2019). Development and validation protocol for an instrument to measure household water insecurity across cultures and ecologies: the Household Water InSecurity Experiences (HWISE) Scale. BMJ Open, 9(1), e023558. doi: 10.1136/bmjopen-2018-023558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuretich RF, & Cerling TE (1983). Hydrogeochemistry of Lake Turkana, Kenya: Mass balance and mineral reactions in an alkaline lake. Geochimica et Cosmochimica Acta, 47(6), 1099–1109. doi: 10.1016/0016-7037(83)90240-5 [DOI] [Google Scholar]
- Zhang H, Fan Y, Xia F, Geng C, Mao C, Jiang S, … Xu Z (2011). Prenatal water deprivation alters brain angiotensin system and dipsogenic changes in the offspring. Brain research, 1382, 128–136. doi: 10.1016/j.brainres.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


