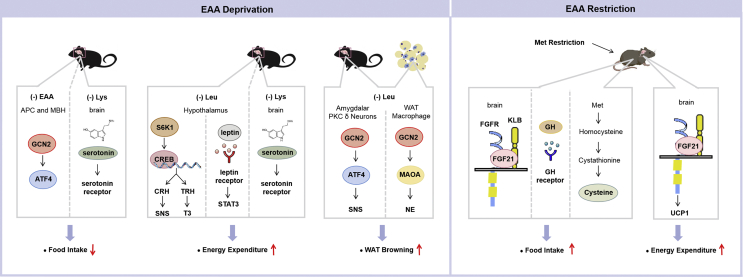
Figure 1.
Mechanisms involved in the regulation of essential amino acid deprivation or restriction on energy balance. Deprivation of a single essential amino acid (EAA) reduces food intake, increases energy expenditure, and induces white adipose tissue (WAT) browning. Activated GCN2 in the anterior piriform cortex (APC) and mediobasal hypothalamus (MBH) is one of the mechanisms mediating animals' response to reject a diet deficient in EAA. The Lys deprivation-decreased food intake and -increased energy expenditure is possibly mediated by enhanced serotonin signaling. Dietary Leu deprivation inhibits hypothalamic S6K1 activity, which in turn increases the activity of cAMP-response element-binding protein (CREB). In the nucleus, corticotrophin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH) transcriptions are stimulated by CREB. After translation and modification, CRH is secreted and activates the sympathetic nervous system (SNS) to stimulate energy expenditure in the whole organism. TRH in turn stimulates the biosynthesis of triiodothyronine (T3) to increase energy expenditure under Leu deprivation. Leptin signaling is also involved in Leu deprivation-increased energy expenditure. Leu deprivation induces WAT browning by GCN2 in PKC-δ neurons of the amygdale and adipose tissue macrophages (ATMs). Activated GCN2 in the amygdale stimulates activating transcription factor 4 (ATF4) expression and increases the PKC-δ neuronal activity to promote WAT browning via the sympathetic nerve; GCN2 activation in ATMs reduces the expression of monoamine oxidase A (MAOA), resulting in increased norepinephrine (NE) secretion from macrophages to adipocytes, and this results in enhanced WAT browning. Met restriction increases energy intake and energy expenditure. These effects are mediated by multiple mechanisms, including fibroblast growth factor (FGF) 21 signaling, growth hormone (GH) signaling, and Met-derived cysteine.