Abstract
Background:
Immune checkpoint inhibitors (ICIs), such as programmed cell death 1 (PD-1) inhibitors, are used to treat multiple cancers. Limited data exist as to the use of ICIs in patients with co-existent interstitial lung disease (ILD). We conducted a retrospective case series to assess clinical and radiological outcomes of subjects with ILD treated with PD-1 inhibitors.
Methods:
Eligible patients were 18 years of age or older, treated with pembrolizumab or nivolumab for oncologic indications, and had evidence of ILD on chest computed tomography (CT) not attributable to radiotherapy prior to initiation of ICI therapy. Outcomes of interest included mortality, hospitalizations for respiratory-related causes, development of pneumonitis, and radiographic change in ILD over a 1-year follow-up period.
Results:
41 subjects were included in the analysis. At one year, 17 subjects (41.5%) were alive, 23 had died (56.1%), and 1 (2.4%) was lost to follow-up. 16 of 23 deaths (69.6%) were due to cancer, 4 (17.4%) due to causes excluding cancer and ILD, and 3 (13.0%) due to hypoxemic respiratory failure from ILD or ICI-induced pneumonitis. Three subjects (7.3%) required hospitalization due to ILD including drug-induced pneumonitis, and 3 (7.3%) developed pneumonitis attributable to anti-PD-1 therapy. On follow-up CT, 32 subjects (78.0%) had stable or improved ILD and 9 (22.0%) had progression of ILD.
Conclusion:
ILD patients receiving PD-1 inhibitors more frequently died of cancer-related causes than from ILD. Further research is needed to determine the safety of ICIs in ILD patients and if ILD subtype may help refine ICI-associated risks.
Keywords: Immunotherapy; Lung Diseases, Interstitial; Idiopathic Pulmonary Fibrosis; Tomography, X-Ray Computed; Carcinomas
MicroAbstract:
This study explores clinical and radiologic outcomes of PD-1 inhibitor use in patients with ILD, who have traditionally been excluded from PD-1 inhibitor safety and efficacy trials. 41 subjects with radiographic evidence of ILD were included in the study. Our findings suggest that PD-1 inhibitors for oncologic indications should not be uniformly withheld in patients with underlying ILD.
Introduction
The discovery of immune checkpoint inhibitors (ICIs) has significantly improved overall survival in patients with advanced cancers. [1] ICIs harness the power of the immune system and redirect it toward tumor elimination. [2] However, this process is imperfect. Infiltration of highly active T-cells into non-malignant tissue can result in local tissue dysfunction and increased morbidity and mortality due to off-target adverse immune effects [3] and need to forgo further ICI therapy. Of these immune-related adverse effects, the development of ICI-related pneumonitis is well-described and can be fatal in a small proportion of patients. [4, 5]
The term interstitial lung disease (ILD) encompasses a heterogenous group of conditions that result in varying degrees of lung inflammation and scarring. While the mechanisms leading to the development of ILD are being further elucidated, the immune system has been implicated in the propagation of fibrosis. [6] Acute exacerbations of ILD are associated with hyper-activation of the immune system and increased mortality. [7] Given the pathological role of inflammation in ILD, it remains uncertain how medications known to promote hyperactivation of the immune system affect patients with ILD.
Limited data exist regarding the use of ICIs in patients with ILD. [8-12] In evaluating the effectiveness of programmed cell death 1(PD-1) inhibitors as cancer therapy, the presence of ILD has been used as an exclusion criterion in some clinical trials. [13-16] Several studies suggest that patients with ILD are at increased risk of developing ICI-related pneumonitis. [11, 12] In contrast, there is recent data to suggest that PD-1 axis inhibition may have a beneficial effect on pulmonary fibrosis when studied in animal models. [17] Thus, more research is needed regarding the safety of ICIs in patients with ILD and associated risks. To address this need, we retrospectively evaluated clinical outcomes and radiographic changes in patients with ILD treated with PD-1 inhibitors for malignancy.
Methods
We performed a retrospective case series of patients with ILD who received PD-1 inhibitors pembrolizumab or nivolumab at Massachusetts General Hospital between September 1, 2014 and June 30, 2018. The Partners Research Patient Data Registry (RPDR), a centralized clinical data registry, was used to identify subjects of interest. A RPDR search was performed to identify subjects that had received pembrolizumab or nivolumab during the specified time frame and had an ILD-related ICD-10 code for respiratory diseases principally affecting the interstitium (J80-J84) or lung diseases due to external agents (J60-J70). Subjects were included if they were 18 years of age or older and had thin-section computed tomography (CT) of the chest performed prior to PD-1 inhibitor treatment and subsequently as part of surveillance of cancer response.
For such subjects, the reports for up to two chest CT scans preceding initiation of PD-1 inhibitors were screened for phrasing reflective of radiologic abnormalities associated with ILD: honeycombing, cystic change, architectural distortion, traction bronchiectasis, reticular opacities, ground glass opacities, and septal thickening. If one or more findings were present in the imaging report, the scan immediately preceding initiation of a PD-1 inhibitor was reviewed by an experienced thoracic radiologist (AS) to confirm presence or absence of ILD. Outside chest CT scans without an associated imaging report were also reviewed by AS. Subjects with changes solely attributed to radiation on imaging report or upon review by AS were excluded. CT features of radiation therapy included architectural distortion, consolidation and traction bronchiectasis in the distribution of a treatment port. Chest CT scans with 1.5mm or thinner slices were reviewed. Subjects with CT evidence of ILD were categorized based on the pre-therapy CT by AS into four CT scanning patterns according to the ATS/ERS/JRS/ALAT guidelines for idiopathic pulmonary fibrosis (IPF) diagnosis. [18] These categories include usual interstitial pneumonia (UIP), probable UIP, indeterminate for UIP, and alternative diagnosis to IPF. For subjects with CT findings consistent with an alternative diagnosis to IPF, the radiological findings and ILD pattern were recorded.
Medical records for each subject were reviewed to obtain relevant medical history and medications prior to initiation of PD-1 inhibitors. Relevant medical history included a prior diagnosis of ILD or the presence of predefined co-morbidities including emphysema or chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), gastroesophageal reflux disease (GERD), and asthma. History of radiation therapy involving the thorax, whether or not related to the cancer for which the patient was receiving treatment, was recorded if received prior to treatment initiation or during the 1-year follow-up period. The most recent pulmonary function tests (PFTs) performed prior to initiation of PD-1 inhibitors were reviewed and the following results recorded if available: forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, and diffusion capacity of lung for carbon monoxide adjusted for hemoglobin (DLCO[Hb]). Treatments recorded included the type, start date, and duration of PD-1 inhibitors, as well as concurrent treatment with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors or use of anti-fibrotic treatment (i.e. pirfenidone or nintedanib) for pulmonary fibrosis. If the exact start date of treatment with PD-1 inhibitors was not found, the duration was calculated using the first day of the stated month for therapy initiation. Additionally, each medical record was reviewed by a rheumatologist (KMD) to determine whether or not subjects met criteria for interstitial pneumonia with autoimmune features (IPAF), [19] given the potential for ICI therapy to worsen underlying autoimmune disease. [20]
The primary clinical outcome of interest, mortality, and secondary outcomes of interest, respiratory-related hospital admissions and development of pneumonitis, were assessed over the course of 1 year after initiation of PD-1 inhibitors. Causes of death and respiratory-related hospitalizations were determined by two separate reviewers (AF and SBM). Causes of death were categorized into 3 categories: ILD-related, cancer-related, and other cause. Cause of death was categorized as ILD-related if there was evidence of worsening underlying ILD or ICI-induced pneumonitis (i.e. respiratory failure and CT findings concerning for or consistent with ILD progression or pneumonitis) or the patient was being treated for an ILD exacerbation or ICI-induced pneumonitis at the time of death. Cause of death was designated cancer-related if no other obvious cause not related to their cancer was identified including progression of ILD or the patient was discharged to hospice for underlying cancer. Subjects were categorized as lost-to-follow-up if no further medical records were found and the subject was not discharged to hospice. In the case of hospital admission, records were reviewed to determine if the cause of hospitalization was related to a respiratory cause. Respiratory-related hospitalization was deemed ILD-related if there was CT evidence of worsening underlying ILD or ICI-induced pneumonitis or the patient was being treated for an ILD exacerbation or ICI-induced pneumonitis. In addition, the incidence of drug-induced pneumonitis was determined by chart review and recorded if there was CT evidence of pneumonitis that resolved on drug cessation or if the subject was clinically treated as having drug-induced pneumonitis with immunosuppression.
To determine the primary radiographic outcome of interest, change in the degree of ILD findings, chest CT images were reviewed over the 1-year follow-up period. AS reviewed both baseline and follow-up CT scans to determine whether the ILD changes were stable (<10% change in findings), progressed (>10% increase in findings), or improved (>10% decrease in findings) based on visual inspection. A mixed response was recorded if more than one CT pattern was present at baseline with different responses at follow-up. For the follow-up scan, the chest CT performed closest to the date of 1-year follow-up after initiation of PD-1 inhibitors was reviewed. If this scan could not be adequately assessed due to superimposed cancer, then the second closest CT scan to the date of 1-year follow-up was reviewed. Outcomes were further stratified by radiological pattern.
Data are reported as mean ± standard deviation or frequency (percentage). A Kaplan-Meier curve was used to explore differences in all-cause mortality rates by radiological pattern (UIP vs all other patterns), and log-rank statistics were used to calculate the hazard ratio, 95% confidence interval (CI), and p-value. A p-value <0.05 was considered statistically significant. Subjects were considered lost to follow-up if no further medical records and no record of death existed. In these cases, subjects were censored at the time of their last medical encounter. Subjects who were alive at the end of the follow-up period were censored at 365 days. Statistical tests were performed using Prism 8.2.0 (GraphPad Software).
Results
377 patients were identified using RPDR as having received PD-1 inhibitor treatment between September 1, 2014 and June 30, 2018 and having an ILD-related ICD-10 code at any point in time (Figure 1). Of these, 65 were excluded due to the absence of chest CT scans prior to initiation of therapy or thereafter as part of cancer surveillance. The remaining patients were screened for abnormalities consistent with ILD by review of CT chest imaging reports. Of these patients, 181 were excluded due to absence of abnormalities suggestive of ILD or abnormalities attributed to prior radiation exposure. The chest CT scans of the remaining 131 patients with abnormalities suggestive of ILD or with CT chest scans without an imaging report were reviewed by AS. Of these, 41 were confirmed to have ILD and were included in our analysis. None of the excluded cases were felt to represent interstitial lung abnormalities (ILAs).
Figure 1:
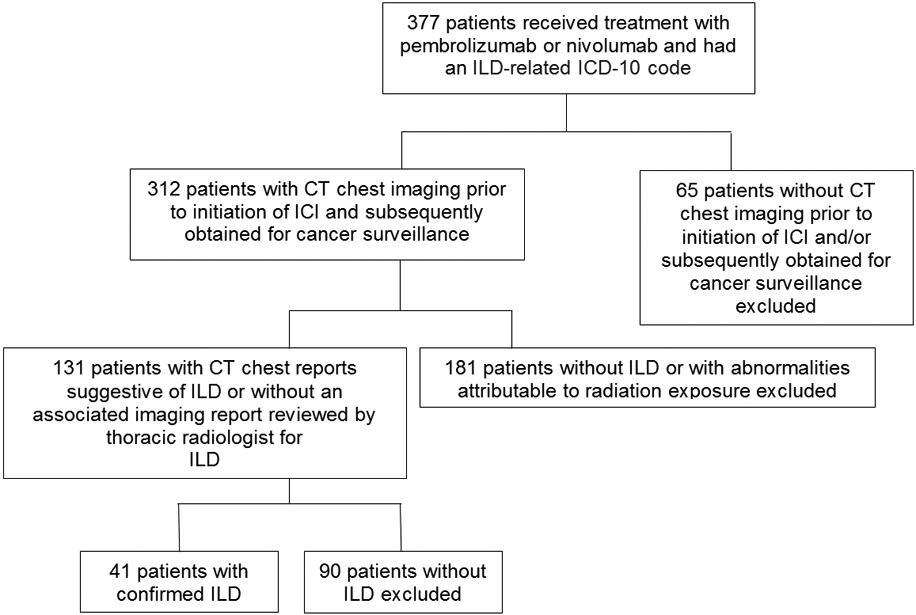
Flow diagram for subjects included.
Baseline demographic and clinical characteristics of the 41 ILD patients are summarized in Table 1. The mean age at time of initiation of immunotherapy was 75 years old. The majority of patients were male (80.5%) and had a previous smoking history (90.2%). Cancer and cancer treatment characteristics are summarized in Table 2. The majority of patients were treated for primary lung cancers (73.2%). Less than half (46.3%) of patients received PD-1 inhibitors for 2 months or less, with majority of discontinuation of therapy due to cancer progression, and only 1 patient received concurrent treatment with a CTLA-4 inhibitor.
Table 1:
Baseline demographic and clinical characteristics at the time of initiation of immune checkpoint inhibitor therapy.
| Characteristic | Patients with ILD (N=41) |
|---|---|
| Age, years (mean ± SD) | 75 ± 9 |
| Male sex (N, %) | 33 (80.5) |
| Current or former smoker (N, %) | 37 (90.2) |
| Comorbidities (N, %) | |
| COPD / emphysema | 11 (26.8) |
| Asthma | 5 (12.2) |
| OSA | 2 (4.9) |
| GERD | 7 (17.1) |
| Autoimmune disease* | 3 (7.3) |
All subjects in the autoimmune disease category had rheumatoid arthritis. Definition of abbreviations: SD = standard deviation; COPD = chronic obstructive pulmonary disease; OSA = obstructive sleep apnea; GERD = gastroesophageal reflux disease.
Table 2:
Cancer and cancer treatment characteristics.
| Characteristic | Patients with ILD (N=41) |
|---|---|
| Primary cancer type, no. (%) | |
| Lung | 30 (73.2) |
| Head & neck | 5 (12.2) |
| Skin | 2 (4.9) |
| Gastrointestinal | 2 (4.9) |
| Renal | 1 (2.4) |
| Hematologic | 1 (2.4) |
| Immunotherapy type, no. (%) | |
| Nivolumab | 26 (63.4) |
| Pembrolizumab | 15 (36.6) |
| Concurrent treatment with CTLA-4 inhibitors, no. (%) | 1 (2.4) |
| Duration of immunotherapy, no. (%) | |
| ≤ 2 months | 19 (46.3) |
| 2-4 months | 5 (12.2) |
| 4-6 months | 3 (7.3) |
| 6-12 months | 7 (17.1) |
| ≥ 12 months | 7 (17.1) |
| Radiation therapy involving the thorax, no. (%) | |
| Prior to immunotherapy | 18 (43.9) |
| During follow-up | 1 (2.4) |
| Total | 19 (46.3) |
ILD-related characteristics are summarized in Table 3. Patients were categorized based on radiologic pattern: 12 patients had a UIP pattern (29.3%), 9 had a probable UIP pattern (21.9%), 12 had an indeterminate for UIP pattern (29.3%), and 8 had an alternative diagnosis pattern (19.5%). Of the 8 with alternative diagnoses, the patterns were as follows: 4 hypersensitivity pneumonitis (HP), 3 organizing pneumonia (OP), and 1 combined respiratory bronchiolitis-associated ILD/OP. Eight patients (19.5%) carried a clinical diagnosis of ILD prior to start of PD-1 inhibitors, and none of the patients were taking anti-fibrotic therapy at the time of ICI initiation. Four patients (9.8%) utilized supplemental oxygen prior to initiation of ICI therapy.
Table 3:
ILD characteristics prior to initiation of immunotherapy.
| Characteristic | Patients with ILD (N=41) |
|---|---|
| Radiological ILD pattern (N, %) | |
| UIP | 12 (29.3) |
| Probable UIP | 9 (21.9) |
| Indeterminate for UIP | 12 (29.3) |
| Alternative Diagnosis (total) | 8 (19.5) |
| HP | 4 (9.8) |
| OP | 3 (7.3) |
| RB/OP | 1 (2.4) |
| RA-ILD* (N, %) | 1 (2.4) |
| IPAF (N, %) | 0 (0.0) |
| Prior diagnosis of ILD at the of ICI initiation | 8 (19.5) |
| Receiving anti-fibrotic therapy** | 0 (0.0) |
| Supplemental O2 requirement | 4 (9.8) |
Rheumatoid arthritis-ILD. No other subjects had ILD in the setting of connective tissue disease.
Treatment with either nintedanib or pirfenidone.
Definition of abbreviations: UIP = usual interstitial pneumonia; HP = hypersensitivity pneumonitis; OP = organizing pneumonia; RB = respiratory bronchiolitis; RA-ILD = rheumatoid arthritis-associated ILD; IPAF = interstitial pneumonia with autoimmune features.
Results of available PFTs prior to ICI initiation are summarized in Table 4. Fifteen patients (36.6%) had FEV1 results, fourteen patients (34.1%) had FVC results, fourteen patients (34.1%) had FEV1/FVC results, and twelve patients (29.3%) had DLCO[Hb] results.
Table 4:
PFT results prior to initiation of immunotherapy
| Parameter | Mean ± SD |
|---|---|
| FEV1 % predicted (n=15) | 78 ± 17.4 |
| FVC % predicted (n=14) | 86.2 ± 18.7 |
| FEV1/FVC % (n=14) | 67.6 ± 10.8 |
| DLCO[Hb] % predicted (n=12) | 59.3 ± 25.8 |
FEV1, FVC, and DLCO[Hb] results reported as percentage of predicted values. Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; DLCO[Hb] = diffusion capacity of lung for carbon monoxide adjusted for hemoglobin
Clinical outcomes of interest during the 1-year follow up period are summarized in Table 5. Seventeen subjects (41.5%) were alive at the end of the follow-up period, 1 (2.4%) was lost to follow-up, and 23 (56.1%) had died. Of the subjects who died, 16 of 23 (69.6%) were deemed related to cancer and 4 (17.4%) attributable to causes other than ILD. The 3 remaining deaths (13.0%) were due to hypoxemic respiratory failure from ILD. For these 3 subjects, we were unable to determine which cases were due to worsening underlying ILD versus ICI-induced pneumonitis given the clinical and radiologic overlap between these two entities. All 3 of the subjects who died from hypoxemic respiratory failure had a UIP pattern on CT. Furthermore, all 3 subjects had a primary lung cancer and had received radiation therapy to the thorax.
Table 5:
Clinical outcomes of interest during the 1-year follow-up period
| Outcome | Patients with ILD (N=41) |
|---|---|
| Mortality | |
| Alive (N, %) | 17 (41.5) |
| Deaths (N, %) | 23 (56.1) |
| Death related to cancer | 16 (39.0) |
| Death due to respiratory failure secondary to ILD or pneumonitis | 3 (7.3) |
| Death due to other causes not related to ILD or cancer* | 4 (9.8) |
| Lost to follow-up (N, %) | 1 (2.4) |
| Hospital admission due to respiratory cause (N, %) | 11 (26.8) |
| Due to underlying ILD or drug-induced pneumonitis | 3 (7.3) |
| Related to other cause** | 8 (19.5) |
| Drug-induced pneumonitis (N, %) | |
| Attributable to immunotherapy | 3 (7.3) |
| Attributable to chemotherapy*** | 1 (2.4) |
Other causes included stroke, dementia, sepsis and gastrointestinal bleeding.
Other causes included COPD exacerbation and pneumonia.
Chemotherapy received was docetaxel.
Eleven subjects (26.8%) were hospitalized for respiratory-related causes, three of which were related to ILD and attributable to pneumonitis (2 to PD-1 inhibitors and 1 to docetaxel). All three subjects with ILD or pneumonitis-related hospitalizations had a primary lung cancer and 2/3 had prior radiation therapy. A total of four subjects developed pneumonitis, three requiring hospitalization as mentioned above and one not requiring hospitalization and attributable to PD-1 inhibitors. Of the three patients who developed PD-1 related pneumonitis, 2 had a UIP pattern on CT and 1 had an alternative diagnosis pattern. 2/3 patients who developed PD-1 related pneumonitis had a primary lung cancer and 2/3 received radiation therapy to the thorax. The patient who developed docetaxel pneumonitis had a primary lung cancer and prior radiation to the thorax.
Radiologic outcomes are summarized in Table 6. The mean duration between baseline and follow-up CT scans was 207 ± 127 days. The majority (78.0%) of patients had stable or improved ILD findings on follow-up imaging while the remaining 22.0% of patients had progression of ILD. Analysis of the UIP subgroup showed that 8 out of 12 of patients had radiographically stable ILD while 4 out of 12 had progression.
Table 6:
Radiological outcomes over the 1-year follow-up period.
| Outcome | ILD pattern | ||||
|---|---|---|---|---|---|
| UIP (N=12) |
Probable UIP (N=9) |
Indeterminate for UIP (N=12) |
Alternative Diagnosis (N=8) |
Total (N=41) |
|
| Change in CT findings (N, %) | |||||
| Stable ILD | 8 (66.7) | 7 (77.8) | 9 (75.0) | 2 (25.0) | 26 (63.4) |
| Improved ILD | 0 (0.0) | 1 (11.1) | 1 (8.3) | 3 (37.5) | 5 (12.2) |
| Progressed ILD | 4 (33.3) | 1 (11.1) | 2 (16.7) | 2 (25.0) | 9 (22.0) |
| Mixed response: stable RB-ILD, improved OP | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (2.4) |
Definition of abbreviations: UIP = usual interstitial pneumonia; RB-ILD = respiratory bronchiolitis-associated interstitial lung disease; OP = organizing pneumonia.
As an exploratory analysis we assessed differences in all-cause mortality by ILD pattern. 75.0% of the UIP subjects (n = 12) had died at 12 months compared to 51.7% of subjects with all other radiographic patterns (n = 29). Patients with a UIP pattern had a trend towards a higher all-cause mortality rate at 1 year (HR = 2.32; 95% CI 0.75, 7.15; p = 0.077) compared to all other ILD subtypes (Figure 2).
Figure 2: Kaplan Meier survival curve for all-cause mortality stratified by ILD pattern.
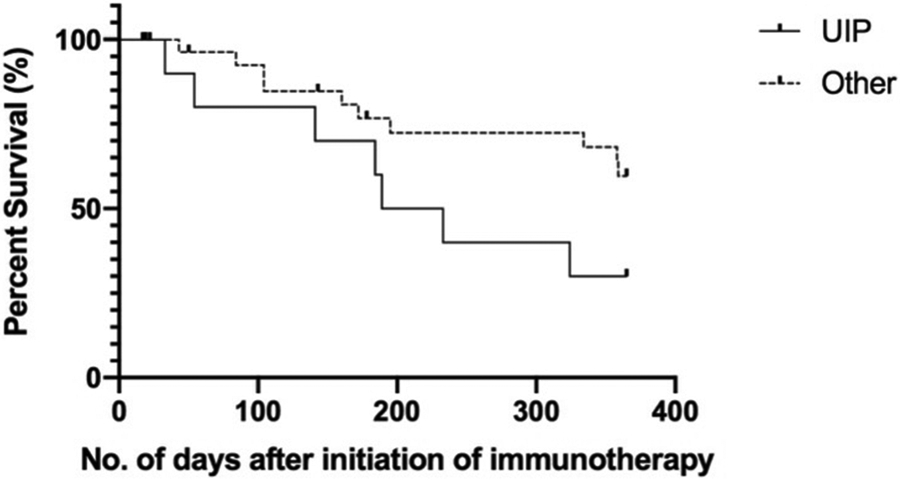
Other includes probable UIP, indeterminate for UIP and alternative diagnosis patterns. HR = 2.318, 95% CI 0.7516 – 7.149, p = 0.077. 75.0% of the UIP subjects (n = 12) had died at 12 months compared to 51.7% of subjects with all other radiographic patterns (n = 29).
Discussion
To the best of our knowledge, this is the first retrospective study to assess clinical and radiographic outcomes of patients with pre-existing CT evidence of ILD who received PD-1 inhibitors. Among 41 patients with ILD treated with ICIs, patients died more frequently from progression of malignancy than progression of ILD or ICI-induced pneumonitis. A minority of patients (13.0%) died from hypoxemic respiratory failure attributable to ILD. These results suggest that for the majority of subjects ICIs did not result in increased ILD-related mortality.
Limited literature exists on the safety of ICIs in ILD patients as many initial clinical trials of ICIs excluded patients with ILD. Recently, a phase II clinical trial of nivolumab was performed in 18 subjects with advanced non-small-cell lung cancer (NSCLC) who had ILD with either a possible or inconsistent with UIP pattern. [8] Of these, 2/18 subjects developed pneumonits which improved with immunosuppression. In a retrospective analysis of 123 subjects with NSCLC who received anti-PD-1 antibiodies, the presence of fibrosis on chest CT was a shown to be a risk factor for anti-PD-1 induced pneumonitis. [12] Furthermore, in a different retrospective study the incidence of pneumonitis was greater in patients with NSCLC receiving ICI therapy who had ILD compared to those without. [11] We report an incidence of 7.3% for PD-1 related pneumonitis over the available follow-up period, and this is higher than what has been previously reported in PD-1 inhibitor clinical trials (2.2%). [3] Our reported incidence of pneumonitis is in keeping with recent statements reporting higher rates in ‘real-world’ settings compared to clinical trials, [4] which excluded patients with ILD. [13-16]
Notably, we detected a trend towards patients with UIP having increased all-cause mortality compared to all other patients with ILD. All deaths due to hypoxemic respiratory failure were in the UIP group. Additionally, two of the three suspected PD-1 inhibitor induced pneumonitis cases were in subjects with a UIP pattern. Prior research has demonstrated an association between a UIP pattern on CT and increased mortality. [21] Among patients with cancer, a greater extent of lung fibrosis on CT in those with lung cancer was shown to be associated with a reduced disease-free survival. [22] In addition, the presence of ILAs was associated with a shorter overall survival in patients with advanced non-small cell lung cancer. [23] Our results raise the possibility that the presence of a UIP pattern may be a risk factor for increased cancer-related mortality and PD-1 inhibitor-induced pneumonitis compared to other forms of ILD; however, further research is needed. Additionally, further studies are needed to determine if prior radiation to the thorax or primary lung cancer are risk factors for the development of ILD exacerbations or PD-1 inhibitor-induced pneumonitis.
Acknowledging the variability in the intervals for CT follow-up, we found that most subjects (78.0%) treated with pembrolizumab or nivolumab had stable or improved ILD findings on follow-up CT scans. For the 22.0% of subjects with progressed radiographic ILD findings, we are unable to determine the potential influence of PD-1 inhibition overlying the natural course of ILD given the lack of a matched control group. Notably, rates of ILD progression based on CT follow-up were higher in the UIP group (33.0%) compared to all other radiographic patterns (17.2%), which is in keeping with the known progressive disease course associated with this radiographic pattern. Given the highly variable CT follow-up times, ranging from 17 to 419 days, and the variability in duration of exposure to anti-PD-1 therapy, our results should be interpreted with caution and should not be used to infer a potential effect of PD-1 inhibitor therapy on underlying ILD.
The majority of subjects had not been diagnosed with ILD at the time of PD-1 inhibitor therapy despite the presence of ILD, which some may classify as ILAs, [24] on CT chest imaging. As such, it is possible that the subjects described herein may represent an ILD subgroup earlier in their disease course, which may have influenced the rates of pneumonitis we detected. Our results should not be extrapolated to inform potential safety of using other ICIs in subjects with ILD as only one patient was treated concurrently with PD-1 and CTLA-4 inhibitors. Furthermore, patients with autoimmune diseases may be at higher risk of ICI immune-related adverse events. [20] Given that there were few patients with autoimmune ILD in our study, we are unable to comment on whether these patients are at higher risk of safety events on ICI therapy.
Our study has several limitations. First, its design was retrospective and thus inherently introduced bias, especially in terms of adjudicating cause of death and hospitalizations. Second, there were differences in the duration of exposure to PD-1 inhibitors for the subjects included. Thus, is it possible that the reported ILD-related complication rates may be underestimated given that over 40% of subjects received PD-1 inhibitors for a duration of 2 months or less. Third, as ICI therapy was mainly given in the setting of advanced cancer during the time frame studied, rates of cancer-related mortality may be overestimated. Fourth, there was substantial variation in the follow-up time of CT chest imaging performed after initiation of ICI therapy, and as such we could be underestimating the rate of ILD progression. In addition, we did not have a control group to serve as a comparator. This was due to inherent difficulties in selecting a control group matched for both advanced cancer and degree of ILD but without exposure to ICIs. Lastly, only a small percentage of subjects without a formal diagnosis of ILD underwent expanded serologic evaluation for an associated etiology of underlying ILD thus limiting the degree of clinical ILD phenotyping.
Conclusion
ILD patients receiving PD-1 inhibitors more frequently died from cancer or non-ILD causes as opposed to worsening underlying ILD or drug-induced pneumonitis. Risk of pneumonitis must be considered when conducting a risk-benefit analysis of prescribing PD-1 inhibitors for cancer treatment in patients with ILD; however, this risk should be weighed against the potential for life-extending oncologic treatment. Given the contribution of aberrant activation of the immune system to ILD pathogenesis and the development of acute exacerbations, a biological rationale exists for further investigation of the effects of ICI therapy on ILD. Ongoing research is needed to expand upon our findings as to the safety and risks of ICI therapy in patients with ILD and to investigate if ILD pattern type can be used to better inform risks of PD-1 inhibitor complications or cancer-related mortality in this patient population.
Clinical Practice Points.
Minimal research exists into the effects of PD-1 inhibitors in patients with ILD receiving immunotherapy for oncologic indications. Many cancer trials assessing the safety and efficacy of PD-1 inhibitors have excluded patients with ILD, and as such, treatment effects in this subpopulation have not been well-studied. From the limited literature available, ILD patients receiving PD-1 inhibitors are at increased risk of pneumonitis when compared to non-ILD patients. Additionally, there exists some hesitancy in prescribing PD-1 inhibitors in this population due to concern that immunotherapy may worsen underlying ILD. We found that ILD patients receiving PD-1 inhibitors more frequently died from cancer or non-ILD related causes compared to worsening underlying ILD or drug-induced pneumonitis. Moreover, in the setting of PD-1 inhibitor use most patients had improved or stable radiographic ILD findings. Our data suggest that PD-1 inhibitors should not be uniformly withheld in patients with ILD and that a thorough risk-benefit analysis that includes pneumonitis risk must be conducted and tailored to the individual patient.
Sources of Funding:
ID received funding for this project from Queen’s University School of Medicine. DO is supported by NIH (T32HL116275). KMD is supported by NIH (T32-AR-007258). DCC is supported by NIH (NCI) U01CA209414. SBM has been supported by funding from the Francis Family Foundation, the Scleroderma Foundation, and NIH (K23HL150331).
Footnotes
Conflicts of Interest: AS has received funding through her institution from Hummingbird Diagnostics. SBM has received funding through her institution from United Therapeutics, Merck, and Promedior and royalties from Wolters Kluwer.
References
- 1.Shen X and Zhao B, Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ, 2018. 362: p. k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A and Wolchok JD, Cancer immunotherapy using checkpoint blockade. Science, 2018. 359(6382): p. 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxi S, Yang A, Gennarelli RL, et al. , Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ, 2018. 360: p. k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sears CR, Peikert T, Possick JD, et al. , Knowledge Gaps and Research Priorities in Immune Checkpoint Inhibitor-related Pneumonitis. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med, 2019. 200(6): p. e31–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidoo J, Wang X, Woo KM, et al. , Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol, 2017. 35(7): p. 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai O, Winkler J, Minasyan M, and Herzog EL, The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front Med (Lausanne), 2018. 5: p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schupp JC, Binder H, Jager B, et al. , Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS One, 2015. 10(1): p. e0116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto D, Yomota M, Sekine A, et al. , Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: A multicenter, open-label single-arm phase II trial. Lung Cancer, 2019. 134: p. 274–278. [DOI] [PubMed] [Google Scholar]
- 9.Byeon S, Cho JH, Jung HA, et al. , PD-1 inhibitors for non-small cell lung cancer patients with special issues: Real-world evidence. Cancer Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiyama N, Honda T, Sema M, et al. , The utility of ground-glass attenuation score for anticancer treatment-related acute exacerbation of interstitial lung disease among lung cancer patients with interstitial lung disease. Int J Clin Oncol, 2020. 25(2): p. 282–291. [DOI] [PubMed] [Google Scholar]
- 11.Shibaki R, Murakami S, Matsumoto Y, et al. , Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother, 2020. 69(1): p. 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi T, Shimizu J, Hasegawa T, et al. , Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer, 2018. 125: p. 212–217. [DOI] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. , Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med, 2015. 373(17): p. 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer J, Reckamp KL, Baas P, et al. , Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med, 2015. 373(2): p. 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horn L, Spigel DR, Vokes EE, et al. , Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol, 2017. 35(35): p. 3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reck M, Rodriguez-Abreu D, Robinson AG, et al. , Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med, 2016. 375(19): p. 1823–1833. [DOI] [PubMed] [Google Scholar]
- 17.Celada LJ, Kropski JA, Herazo-Maya JD, et al. , PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Sci Transl Med, 2018. 10(460). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghu G, Remy-Jardin M, Myers JL, et al. , Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med, 2018. 198(5): p. e44–e68. [DOI] [PubMed] [Google Scholar]
- 19.Fischer A, Antoniou KM, Brown KK, et al. , An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J, 2015. 46(4): p. 976–87. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Wahab N, Shah M, Lopez-Olivo MA, and Suarez-Almazor ME, Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med, 2018. 168(2): p. 121–130. [DOI] [PubMed] [Google Scholar]
- 21.Putman RK, Gudmundsson G, Axelsson GT, et al. , Imaging Patterns Are Associated with Interstitial Lung Abnormality Progression and Mortality. Am J Respir Crit Care Med, 2019. 200(2): p. 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasawa T, Okudela K, Takemura T, et al. , Computer-aided Quantification of Pulmonary Fibrosis in Patients with Lung Cancer: Relationship to Disease-free Survival. Radiology, 2019. 292(2): p. 489–498. [DOI] [PubMed] [Google Scholar]
- 23.Araki T, Dahlberg SE, Hida T, et al. , Interstitial lung abnormality in stage IV non-small cell lung cancer: A validation study for the association with poor clinical outcome. Eur J Radiol Open, 2019. 6: p. 128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatabu H, Hunninghake GM, Richeldi L, et al. , Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med, 2020. 8(7): p. 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]


