Abstract
Background and Objectives
Although there is evidence of disruption in acute cerebrovascular and cardiovascular care during the coronavirus disease 2019 (COVID-19) pandemic, its downstream effect in primary care is less clear. We investigated how the pandemic affected utilization of cerebrovascular and cardiovascular care in general practices (GPs) and determined changes in GP-recorded diagnoses of selected cerebrovascular and cardiovascular outcomes.
Methods
From electronic health records of 166,929 primary care patients aged 30 or over within the Rotterdam region, the Netherlands, we extracted the number of consultations related to cerebrovascular and cardiovascular care, and first diagnoses of selected cerebrovascular and cardiovascular risk factors (hypertension, diabetes, lipid disorders), conditions, and events (angina, atrial fibrillation, TIA, myocardial infarction, stroke). We quantified changes in those outcomes during the first COVID-19 wave (March–May 2020) and thereafter (June–December 2020) by comparing them to the same period in 2016–2019. We also estimated the number of potentially missed diagnoses for each outcome.
Results
The number of GP consultations related to cerebrovascular and cardiovascular care declined by 38% (0.62, 95% confidence interval 0.56–0.68) during the first wave, as compared to expected counts based on prepandemic levels. Substantial declines in the number of new diagnoses were observed for cerebrovascular events: 37% for TIA (0.63, 0.41–0.96) and 29% for stroke (0.71, 0.59–0.84), while no significant changes were observed for cardiovascular events (myocardial infarction [0.91, 0.74–1.14], angina [0.77, 0.48–1.25]). The counts across individual diagnoses recovered following June 2020, but the number of GP consultations related to cerebrovascular and cardiovascular care remained lower than expected throughout the June to December period (0.93, 0.88–0.98).
Discussion
While new diagnoses of acute cardiovascular events remained stable during the COVID-19 pandemic, diagnoses of cerebrovascular events declined substantially compared to prepandemic levels, possibly due to incorrect perception of risk by patients. These findings emphasize the need to improve symptom recognition of cerebrovascular events among the general public and to encourage urgent presentation despite any physical distancing measures.
There has been considerable interest in the effect of coronavirus disease 2019 (COVID-19) on specialized cerebrovascular and cardiovascular health care. There was a global decline of 11.5% in stroke hospital admissions1 and the number of hospital admissions for acute coronary syndromes in England fell by 40% in March 2020.2 Besides acute care, the pandemic is likely to have long-lasting and serious implications also for primary care. As health care resources were allocated to COVID-19 care, the use and provision of non-COVID-19 primary care were inevitably affected. In addition, people may have delayed seeking help, either for fear of infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or to avoid burdening health care providers. The risk of severe health complications due to delays or failure in seeking medical attention increases when risk factors or warning signs of underlying diseases are left unheeded.3 This is particularly relevant for preventive care, or for symptoms that have been considered unimportant by patients due to incorrect perception of risk, as seems particularly the case for transient cerebrovascular symptoms.4
This population-based study complements the existing evidence on acute care and examines how the COVID-19 pandemic affected utilization of cerebrovascular and cardiovascular care in general practices (GPs). We leveraged data from primary care consultations to quantify the overall changes in cerebrovascular and cardiovascular care during the COVID-19 pandemic and accompanying physical distancing measures taken by national governments. We also determined changes in the number of diagnoses of individual cerebrovascular and cardiovascular risk factors and events as registered by general practitioners. Finally, we estimated the number of cerebrovascular and cardiovascular diagnoses among community-dwelling individuals that may have been missed during the pandemic.
Methods
Data Sources and Study Population
We conducted a population-based cohort study using the Rijnmond Primary Care database, a region-specific derivative of the Integrated Primary Care Information database,5 managed by the Department of General Practice of the Erasmus MC–University Medical Centre Rotterdam. The data cover information about approximately 18% of the population of the greater Rotterdam area in the Netherlands, equally distributed across the region. The database contains information about patients and episodes of care routinely collected by general practitioners: diagnoses, symptoms, clinical findings, test results, drug prescriptions, and other relevant information. The Rotterdam region is a dense urban area; 36% of the residents have non-Dutch background, of which 70% have non-Western background.6 The average distance to the nearest GP practice is 0.6 kilometers (0.37 miles).7 The study period started on 1 January 2016 and ended on 31 December 2020.
We included all patients aged 30 years and over. A patient's period of eligibility started on January 1, 2016; 1 year from registration within a GP; date of turning 30 years of age; or from the date of the inclusion of the GP in Rijnmond Primary Care database (whichever came later). The eligibility period ended by patient's death, transferring to another practice, the end of data collection from the practice, or on December 31, 2020 (whichever came first).
The study was carried out following the RECORD guidelines.8
Outcomes and Procedures
We focused on 2 types of outcomes: (1) the overall number of GP consultations related to cerebrovascular and cardiovascular care and (2) new diagnoses of specific risk factors (hypertension, type 2 diabetes, and lipid disorders) and cerebrovascular and cardiovascular events (myocardial infarction [MI], angina pectoris, atrial fibrillation, stroke, and TIA).9
A GP consultation was defined as any contact between a GP and a patient (in person, by phone, or online), in which symptoms, complaints, diagnosis, or treatment were discussed. The first diagnosis was defined as a GP consultation, for which a GP entered a new diagnosis in the patient's record, previously not recorded in the patient's history. To include also patients who were diagnosed in hospital or during out-of-hours services of primary care, we also included diagnosis records based on correspondence between GPs and specialist care providers.
The selected diagnoses were identified in the patients' records based on a country-specific version of the International Classification of Primary Care. The classification is managed by the Dutch College of General Practitioners10 and is adopted by all Dutch GPs (see Supplementary materials, eTable 1, links.lww.com/WNL/B688).
Data Analysis
The analyses were conducted in 4 steps. First, we extracted the total number of GP consultations related to all cerebrovascular and cardiovascular diagnoses and symptoms for each month in the study period and the monthly number of new diagnoses for each individual outcome of interest. The sample size was calculated for each month and each individual outcome (the start date of the eligibility period was set on the first day of the month following the exact start date; the end date was set on the last day of the month in which the eligibility period ended). Patients who were already diagnosed with one of the selected outcomes were excluded from the corresponding analyses following their date of diagnosis. Patients with a history of any of the outcomes of interest before study entry were excluded from the sample for this particular outcome.
Second, we fitted a negative binomial regression model to the monthly counts of GP consultations and separately to monthly counts of first diagnosis for each outcome of interest. We tested for overdispersion in our data and used Poisson regression for outcomes in which we did not detect statistically significant overdispersion. The seasonal pattern of the data was modeled with a categorical variable indicating a calendar month for each data point; a possible long-term linear trend in the outcomes was modeled with a covariate indicating the number of months since the start of the study.
Third, we conducted segmented time series analyses to quantify the level change following the implementation of governmental COVID-19 control measures. We included 2 with-restriction variables in the model that divided the data into 3 segments: the pre-COVID-19 period (January 2016–February 2020), the period of the first lockdown in the Netherlands (March–May 2020), and the period after the first lockdown (June–December 2020). The first variable defined the period of the first lockdown and was set to 0 for all months in the study, except for March, April, and May 2020. Since the nationwide lockdown in the Netherlands was introduced on March 11 and ended on May 10, 2020, these 2 months were considered transition periods with only a partial impact of the pandemic. The first with-restriction value was therefore set to 1 for April and to 0.7 for March and May 2020. The value of 0.7 provided the best fit to the data, based on the root square error, across all outcomes. The second with-restriction variable defined the period following the spring lockdown; it was set to 0 for all months before June 2020 and to 1 for all months afterwards (June–December 2020). The estimated coefficients of the 2 with-restriction variables defined the effect of the pandemic throughout 2020: the first with-restriction coefficient represents the relative decline in the monthly counts as a proportion of expected counts during the first COVID-19 wave, the second with-restriction coefficient estimated the relative decline in the monthly counts in the period after the first COVID-19 wave (values less than 1 indicate a decline and values greater than 1 indicate an increase.) To account for heteroscedasticity and autocorrelation in the error terms, the standard errors of the estimates and the coefficients were calculated using heteroscedasticity and autocorrelation consistent covariance matrix estimation.11 The natural logarithm of the sample size was used as an offset to control for the changing sample size throughout the study period. Detailed specification of the model is available in the Supplementary material (eAppendix 1, links.lww.com/WNL/B688).
Fourth, to estimate the absolute effect on the number of new diagnoses, we fitted the same model again, but with the 2 with-restriction variables set to zero for all months. The fitted values of the model for March through December 2020 thus represented the expected counts in the counterfactual scenario where no pandemic and no restrictions took place. By comparing these expected counts with observed counts for each calendar month from March to December 2020, we estimated the cumulative number of potentially missed or delayed diagnoses.
To show how the observed counts during the pandemic period differed from those observed during the prepandemic period, we plotted the observed counts for each calendar month in 2020 against observed counts averaged over 2 prepandemic periods: 2018–2019 and 2016–2019 (eFigure 1.1 and eFigure 1.2, Supplementary material, links.lww.com/WNL/B688).
We conducted a sensitivity analysis to assess the robustness of our findings. We reanalyzed the data in which we set the first with-restriction value for March and May 2020 to 0.2, 0.4, 0.6, and 0.8 in order to account for potential variation of the lockdown impact on the main results.
Data extraction and analyses were carried out in R (version 4.0.0),12 data were prepared and plotted using the tidyverse collection of packages,13 and the negative binomial regression model was fitted using the MASS package.14
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Governance Board of Rijnmond Primary Care (project number 2020.012). All patient data are deidentified for research purposes; no patient consent was therefore required.
Data Availability
Due to legal restraints, data are not made publicly available in a repository. Access to the data will be provided upon reasonable request and subject to approval by the Governance Board of Rijnmond Primary Care.
Results
Population Characteristics
The total study population (patients over 30 years of age) grew from 122,302 patients in January 2016 to 166,929 in December 2020, as more GP practices were included in the database. The average age of the patients was 55.7 years; 51% of the population were female. The population characteristics remained stable throughout the study period. Detailed characteristics of the study population and the relevant subgroups are available in the Supplementary material (eTable 2, eTable 3, links.lww.com/WNL/B688).
The Netherlands experienced 2 COVID-19 waves in 2020. The first wave peaked on March 27, 2020, with 620 daily hospital admissions (3.56 per 100,000 people); the second wave had 2 peaks, the first on November 2, 2020, with 399 daily hospital admissions (2.29 per 100,000 people), and the second on December 28, 2020, with 469 daily hospital admissions (2.69 per 100,000 people).15 The Rotterdam region recorded 47 daily hospital admissions (3.21 per 100,000 people) at the peak of the first wave, 53 daily admissions at the first peak of the second wave (3.62 per 100,000 people), and 37 daily admissions at the second peak (2.53 per 100,000 people).16
Changes in the Total Number of GP Consultations Related to Cerebrovascular and Cardiovascular Care
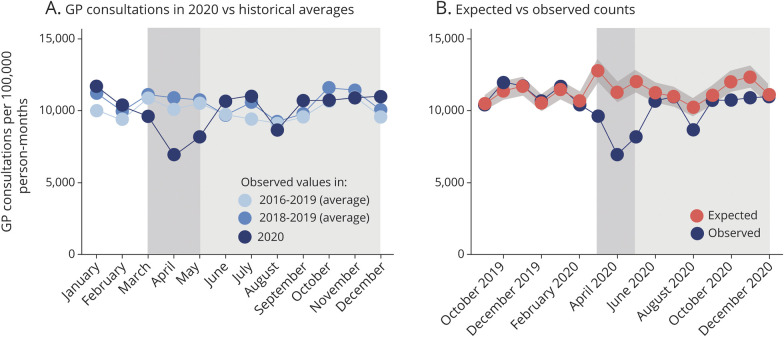
Immediately after the start of the first national lockdown in the Netherlands, the number of GP consultations related to cerebrovascular and cardiovascular care declined to 62% of the expected count (0.62; 95% confidence interval 0.56–0.68). After the initial steep decline, the number of consultations returned closer to prepandemic level, but remained lower than the expected number throughout the rest of 2020 (0.93; 0.88–0.98) (Figure 1).
Figure 1. All General Practice Consultations on Cardiovascular and Cerebrovascular Conditions.
(A) Number of general practice (GP) consultations related to cardiovascular care in 2020 compared to historical averages. (B) Number of GP consultations related to cardiovascular care in 2020 compared to estimated expected number of GP consultations. The shaded area indicates the pandemic period with the period of the first lockdown in the Netherlands (March 15 to May 11, 2020) highlighted.
The decline in GP consultations was higher for women than for men: 0.59 (0.54–0.66) vs 0.64 (0.59–0.71). Greater declines were observed for older adults: the largest relative decline was observed for people between 66 and 75 years of age (0.58; 0.52–0.65); the smallest decline was observed for the youngest group in this study (31–45 years) (0.74; 0.65–0.85). The youngest group is the only age group for which we observed a statistically significant increase in the number of consultations in the second half of 2020 (1.08; 1.00–1.17) as compared to the expected counts (see Supplementary material, eFigure 2, eFigure 3, links.lww.com/WNL/B688).
Changes in the Number of New Diagnoses Across Cerebrovascular and Cardiovascular Events and Risk Factors
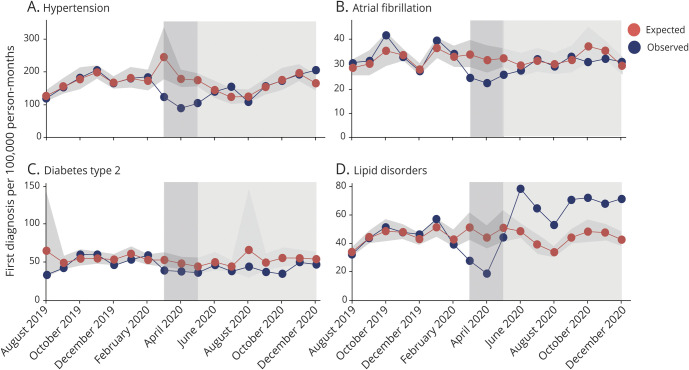
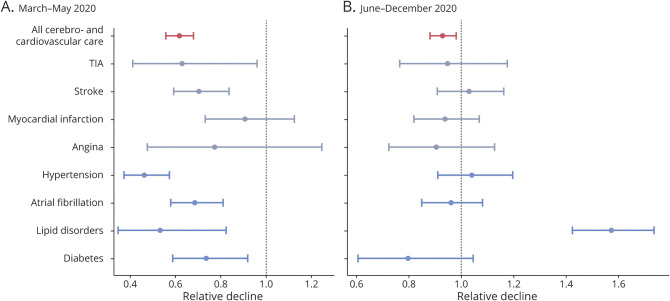
While the number of first diagnoses for cerebrovascular events declined at the start of the spring lockdown in the Netherlands (TIA 0.63 [0.41–0.96], stroke 0.71 [0.59–0.84]), the number of first diagnoses for cardiovascular events did not change significantly (MI 0.91 [0.73–1.13], angina pectoris 0.77 [0.48–1.25]. Significant changes were also observed for risk factors (hypertension 0.47 [0.38–0.57], type 2 diabetes 0.74 [0.59–0.92], lipid disorders 0.54 [0.35–0.82]) and for atrial fibrillation (0.69 [0.58–0.81]). The observed monthly numbers of new diagnoses for each outcome in 2020, compared to the estimated mean expected numbers per 100,000 person-months, is represented in Figure 2 and Figure 3. The relative decline for each outcome as compared to expected levels is presented in Figure 4. Sensitivity analysis using varying values for the first with-restriction variable for the 2 transition months (March and May 2020) provided consistent results for all outcomes (see Supplementary material, eTable 4, links.lww.com/WNL/B688).
Figure 2. Observed vs Expected Monthly Number of New Diagnoses: Cardiovascular and Cerebrovascular Events.
Number of new diagnoses of cerebrovascular ([A] TIA, [B] stroke) and cardiovascular events ([C] myocardial infarction, [D] angina): observed and expected counts of new diagnoses (August 2019–December 2020). The shaded area indicates the pandemic period with the period of the first lockdown in the Netherlands (March 15, 2020–May 11, 2020) highlighted.
Figure 3. Observed vs Expected Monthly Number of New Diagnoses: Risk Factors.
Number of new diagnoses for (A) hypertension, (B) atrial fibrillation, (C) type 2 diabetes, and (D) lipid disorders: observed and expected counts of new diagnoses (August 2019 to December 2020). The shaded area indicates the pandemic period with the period of the first lockdown in the Netherlands (15 March to 11 May 2020) highlighted.
Figure 4. Relative Decline in the Number of First Diagnoses.
Relative decline in the number of first diagnoses for all cerebrovascular and cardiovascular general practice consultations and for individual outcomes compared to expected numbers. (A) March to May 2020; (B) June to December 2020.
From June 2020 onwards, the monthly counts of first diagnoses increased and returned to their pre-COVID-19 levels, but, with the exception of lipid disorders, did not exceed the expected counts. The rate of new diagnosis for lipid disorders exceeded significantly the expected rate for all months from June 2020 onwards, with a rate of change of 1.57 (1.43–1.74) (Figure 4B).
Atrial fibrillation and stroke were the only diagnoses with differences between men and women during the lockdown in March, April, and May 2020. A decline was observed among women for atrial fibrillation (0.53, 0.41–0.68), whereas no significant change was observed among men (0.82, 0.64–1.06). For stroke, the change for women was 0.52 (0.42–0.66) and 0.88 (0.62–1.26) for men (see Supplementary material, eFigure 4.1, links.lww.com/WNL/B688).
Number of Potentially Missed Cerebrovascular and Cardiovascular Diagnoses in Primary Care
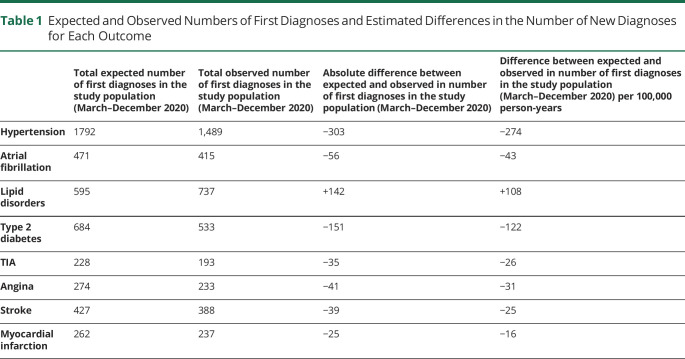
The effect of the COVID-19 pandemic on cerebrovascular and cardiovascular care in terms of number of potentially missed cases for each diagnosis is presented in Table 1. These numbers reflect the difference between the observed number of first diagnoses during the COVID-19 pandemic and the predicted number of first diagnoses, based on our model estimates. The highest number of potentially missed cases was for hypertension with 303 fewer diagnoses in the study population from March to December 2020 (328 diagnoses per 100,000 person-years). There were 39 fewer diagnoses of stroke and 35 fewer diagnoses of TIA in the study population (25 and 26 diagnoses per 100,000 person-years, respectively). Contrary to all the other outcomes, the number of new diagnoses of lipid disorders increased by >100 diagnoses per 100,000 person-years as compared to the expected counts.
Table 1.
Expected and Observed Numbers of First Diagnoses and Estimated Differences in the Number of New Diagnoses for Each Outcome
Discussion
The overall number of GP consultations related to cerebrovascular and cardiovascular care dropped by 38% during the first lockdown and remained below prepandemic levels in the second half of 2020. The number of new diagnoses of cerebrovascular events declined substantially, in contrast with the number of new diagnoses of acute cardiovascular events, which remained stable. This suggests that patients may be less alarmed by symptoms suggestive of TIA or stroke (as compared to MI) and therefore less likely to contact their GPs.4 This behavior might be further reinforced during lockdowns when people with cerebrovascular symptoms may avoid seeking care due to fear of COVID-19 and related complications, especially when public authorities urge them to stay home.17
Previous studies showed a substantial decline in GP consultations across a range of diagnoses and health conditions during the first half of 2020, including acute cerebrovascular and cardiovascular events.18-20 Qualitative studies also documented disruption to primary care across a range of European countries.21 A global observational study found 11.5% decline in stroke admissions (including TIA) during the initial 4 months of the pandemic (March–June 2020),1 which is in line with our finding. A United Kingdom–wide study focusing on missed GP contacts for acute events found decline by 0.63 in the number of contacts for TIA in March 2020 and 0.59 for strokes. Contrary to our study, the authors also observed a substantial decline in the number of GP contacts for MI, unstable angina, and diabetes emergencies.20
The number of potentially missed diagnoses of stroke, TIA, atrial fibrillation, and risk factors may lead to increased health complications. The risk of recurrent stroke for people after their first stroke is estimated to be 11% within 1 year and 26% within 5 years.22 It has been suggested that timely secondary preventive measures can reduce the risk of recurrent cardiovascular events by up to 70%.3,23 Patients who did not seek medical attention during the pandemic for cerebrovascular symptoms, and hence did not start risk-lowering treatment, may therefore be at increased risk of repeat neurologic events and death. The composite risk of stroke, acute coronary syndrome, or death from cardiovascular causes at 5 years after TIA is estimated to be nearly twice as high for people without any risk-reduction measures.3
The high number of potentially missed diagnoses of cerebrovascular and cardiovascular risk factors and atrial fibrillation is worrying, given the established benefits of preventive interventions to reduce the risk of cerebrovascular and cardiovascular events and mortality.24,25 Hypertension is the only outcome we studied in which the effect of the pandemic was detectable at the level of individual GPs: the total potentially missed diagnoses of hypertension equaled around 8 cases per year per general practitioner (with an average number of patients per GP in the Netherlands being 2,300).26
Limitations of our study include possible inconsistent recording practices among GPs. Furthermore, interventions at the level of individual GP practices might have influenced our data and our results. We also did not include recurrent events, which may follow different patterns of health-seeking behavior as those who have already experienced a cerebrovascular or cardiovascular event might be more likely to recognize their symptoms. We also could not distinguish between patients diagnosed by GPs and patients diagnosed in hospital/emergency departments (who were later referred to their GP). It is possible that the effect of the pandemic was different for these 2 groups of patients. Finally, we did not have access to data on education levels, smoking, and other lifestyle indicators, which might have provided greater insight into health care avoidance patterns.
A key strength of this study is that we assessed the effect of COVID-19 on primary care by the inclusion of a broad spectrum of cerebrovascular and cardiovascular conditions, also including risk factors. As our data covered the full year of 2020, we were also able to examine changes in health care seeking behavior over a longer time span.
The COVID-19 pandemic has a potentially long-lasting effect on the prognosis of cerebrovascular and cardiovascular diseases due to missing or delayed diagnosing of risk factors and most notably cerebrovascular events. Even though the number of new diagnoses returned to the expected levels in the second half of 2020, the number of potentially missed diagnoses during the first wave were not recuperated. The decline in the number of first diagnoses of stroke and TIA calls for improvement of symptom recognition of cerebrovascular events among the general public and for awareness campaigns to encourage urgent presentation despite any existing physical distancing measures.
The challenge for the near future is to identify patients who avoided health care and develop strategies for cerebrovascular and cardiovascular primary care that will minimize the consequences of undiagnosed risk factors and events during the COVID-19 pandemic. A critical question is to assess whether this disruption in primary care will lead to increased cerebrovascular and cardiovascular morbidity and mortality in the future.
Glossary
- COVID-19
coronavirus disease 2019
- GP
general practice
- MI
myocardial infarction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Appendix. Authors

Footnotes
Editorial, page 219
Study Funding
This work was funded by the Dutch organization for health research and health innovation (ZonMw), project number 10430022010016. P.V., E.I.T.d.S., and P.J.E.B. had full access to the data reported in the study. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosure
Some authors report receiving consulting fees and research support from health care companies, government agencies, and foundations. Go to Neurology.org/N for full disclosures.
References
- 1.Nogueira RG, Qureshi MM, Abdalkader M, et al. . Global impact of COVID-19 on stroke care and intravenous thrombolysis. Neurology. 2021;96(23):e2824-e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mafham MM, Spata E, Goldacre R, et al. . COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarenco P, Lavallée PC, Monteiro Tavares L, et al. . Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378(23):2182-2190. [DOI] [PubMed] [Google Scholar]
- 4.Wolters FJ, Li L, Gutnikov SA, Mehta Z, Rothwell PM. Medical attention seeking after transient ischemic attack and minor stroke before and after the UK Face, Arm, Speech, Time (FAST) public education campaign: results from the oxford vascular study. JAMA Neurol. 2018;75(10):1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlug AE, van der Lei J, Mosseveld BM, et al. . Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med. 1999;38(4-5):339-344. [PubMed] [Google Scholar]
- 6.Bevolking; Leeftijd, Migratieachtergrond, Geslacht, Regio, 1 Jan. 1996-2020 [online]. Accessed September 30, 2021. opendata.cbs.nl/#/CBS/nl/dataset/37713/table [Google Scholar]
- 7.StatLine. Huisarts in 2019 Gemiddeld Op 1 Kilometer Afstand [online]. Accessed September 30, 2021. cbs.nl/nl-nl/nieuws/2020/17/huisarts-in-2019-gemiddeld-op-1-kilometer-afstand [Google Scholar]
- 8.Benchimol EI, Smeeth L, Guttmann A, et al. . The Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement. PLOS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leening MJ, Kavousi M, Heeringa J, et al. . Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27(3):173-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genootschap NH. NHG-tabel 24-ICPC-Versie 8. 2020. [Google Scholar]
- 11.Zeileis A. Object-oriented computation of sandwich estimators. J Stat Softw. 2006;1(9):2006. [Google Scholar]
- 12.R: A Language and Environment for Statistical Computing [computer program]. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 13.Wickham H, Averick M, Bryan J, et al. . Welcome to the tidyverse. J Open Source Softw. 2019;4. [Google Scholar]
- 14.VW N, RB D. Modern Applied Statistics with S. 4th ed. Springer; 2002. [Google Scholar]
- 15.Ontwikkeling COVID-19 in grafieken [online]. Accessed September 30, 2021. rivm.nl/coronavirus-covid-19/grafieken [Google Scholar]
- 16.Gemeld aantal ziekenhuisopnames op één dag van inwoners van veiligheidsregio Rotterdam-Rijnmond [online]. Accessed September 30, 2021. coronadashboard.rijksoverheid.nl/veiligheidsregio/VR17/ziekenhuis-opnames [Google Scholar]
- 17.Henrique D, Pedro SCM, Sheila COM, et al. . Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke. 2020;51(8):2315-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams R, Jenkins DA, Ashcroft DM, et al. . Diagnosis of physical and mental health conditions in primary care during the COVID-19 pandemic: a retrospective cohort study. Lancet Public Health. 2020;5(10):e543-e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr MJ, Steeg S, Webb RT, et al. . Effects of the COVID-19 pandemic on primary care-recorded mental illness and self-harm episodes in the UK: a population-based cohort study. Lancet Public Health. 2021;6(2):e124-e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansfield KE, Mathur R, Tazare J, et al. . Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digital Health. 2021;3(4):e217-e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanat M, Hoste M, Gobat N, et al. . Transformation of primary care during the COVID-19 pandemic: experiences of healthcare professionals in eight European countries. Br J Gen Pract. 2021;71(709):e634-e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keerthi MM, Charles DAW, Anthony GR, Peter UH, Peter LK-R, Andrew PG. Risk and cumulative risk of stroke recurrence. Stroke. 2011;42(5):1489-1494. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen RA, Petursson H, Hetlevik I. Stroke follow-up in primary care: a prospective cohort study on guideline adherence. BMC Fam Pract. 2018;19(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blood Pressure Lowering Treatment Trialists Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397(10285):1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruff CT, Giugliano RP, Braunwald E, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. [DOI] [PubMed] [Google Scholar]
- 26.Kringos DS, Boerma WGW. Building primary care in a changing Europe: Case studies, Observatory Studies Series; Vol 40, 20 ed. European Observatory on Health Systems and Policies; 2015. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to legal restraints, data are not made publicly available in a repository. Access to the data will be provided upon reasonable request and subject to approval by the Governance Board of Rijnmond Primary Care.