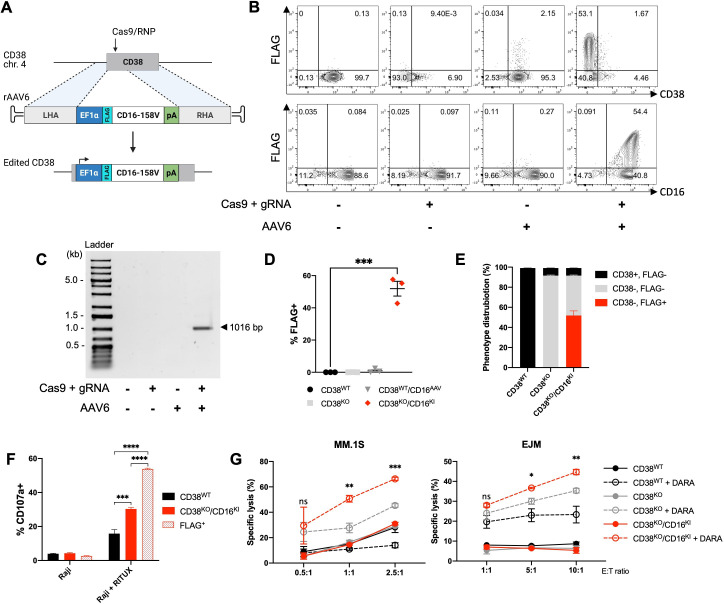
Figure 6.
CD16-158V gene insertion coupled with CD38 knockout enhances CD38-targeted ADCC. (A) Gene knock-in (KI) strategy to insert a CD16-158V cassette containing a FLAG tag and EF1α promoter into the CD38 locus using Cas9/RNP and rAAV6. (B) Representative flow cytometry plots of CD38, FLAG, and CD16 expression in NK cells 7 days after gene KI targeted to the CD38 locus. (C) Representative gel electrophoresis image of PCR amplification products to detect site-specific integration of the CD16-158V transgene within the CD38 locus. (D) FLAG expression assessed by percent positive NK cells 7 days following Cas9/RNP electroporation and/or rAAV6 infection examined by flow cytometry (n=4 donors). (E) Phenotype distribution of CD38 and FLAG in control and edited NK cells, examined by flow cytometry (n=4 donors). (F) NK cell degranulation (measured by CD107a) by CD38KO/CD16KI NK cells compared with unedited control NK cells following coculture with Raji cells with or without rituximab (RITUX). CD107a was gated on bulk and FLAG-positive CD38KO/CD16KI NK cells (n=3 donors). (G) DARA-mediated ADCC by CD38KO/CD16KI NK cells compared with CD38KO and control NK cells assessed against MM.1S and EJM multiple myeloma target cells at various effector-to-target (E:T) ratios (n=3 donors). Statistics determined with the Student’s t-test, two tailed, ns=not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. ADCC, antibody-dependent cellular cytotoxicity; DARA, daratumumab; KO, knockout; WT, wild type; MM, multiple myeloma; NK, natural killer; LHA, left homology arm; RHA, right homology arm.