Summary
Psychedelics are compounds that alter consciousness by acting on serotonin receptors in the brain. The term ‘psychedelic’, from the Greek for mind manifesting, refers to the drugs’ subjective effects and was first proposed by Humphry Osmond in 1956. Other terms have been used to emphasize different aspects of the psychological experiences produced by various related compounds, including hallucinogens (perceptual), entheogens (spiritual), and empathogens or entactogens (social-emotional). The diversity in terminology reflects the existence of hundreds of potential psychedelic compounds with a spectrum of behavioral and neurobiological effects. Recent data on the effectiveness of psychedelics for treating mental illnesses has led to a resurgence of interest in their neurobiological effects. The purpose of this Primer is to provide those interested in the field of psychedelics with a concise and accessible overview of the scientific data.
Chemistry
Psychedelics can be divided into three classes based on their chemical structure: tryptamines, ergolines, and phenethylamines (Figure 1). Tryptamines are characterized by an indole, which is a 6-member benzene ring fused to a 5-member pyrrole ring with an ethylamine chain at the C3 position. Addition of methyl groups to the ethylamine chain and different functional groups at other positions, e.g., C4 and C5, yields psilocybin, psilocin (the active metabolite of psilocybin), DMT, and 5-MeO-DMT (see Figure 1 for full names). These compounds are closely related to the endogenous neurotransmitter serotonin (also 5-hydroxytryptamine, or 5-HT), which is a tryptamine with a hydroxyl group at the C5 position. Ergolines, initially isolated from the ergot fungus and then further processed via chemical reactions, include LSD. The phenethylamine class, based on a scaffold of a benzene ring with an amino group attached through two-carbon, includes 2C-B, mescaline, amphetamine analogues such as DOI and DOM, and derivatives such as 25I-NBOMe.
Figure 1: Psychedelic compounds.
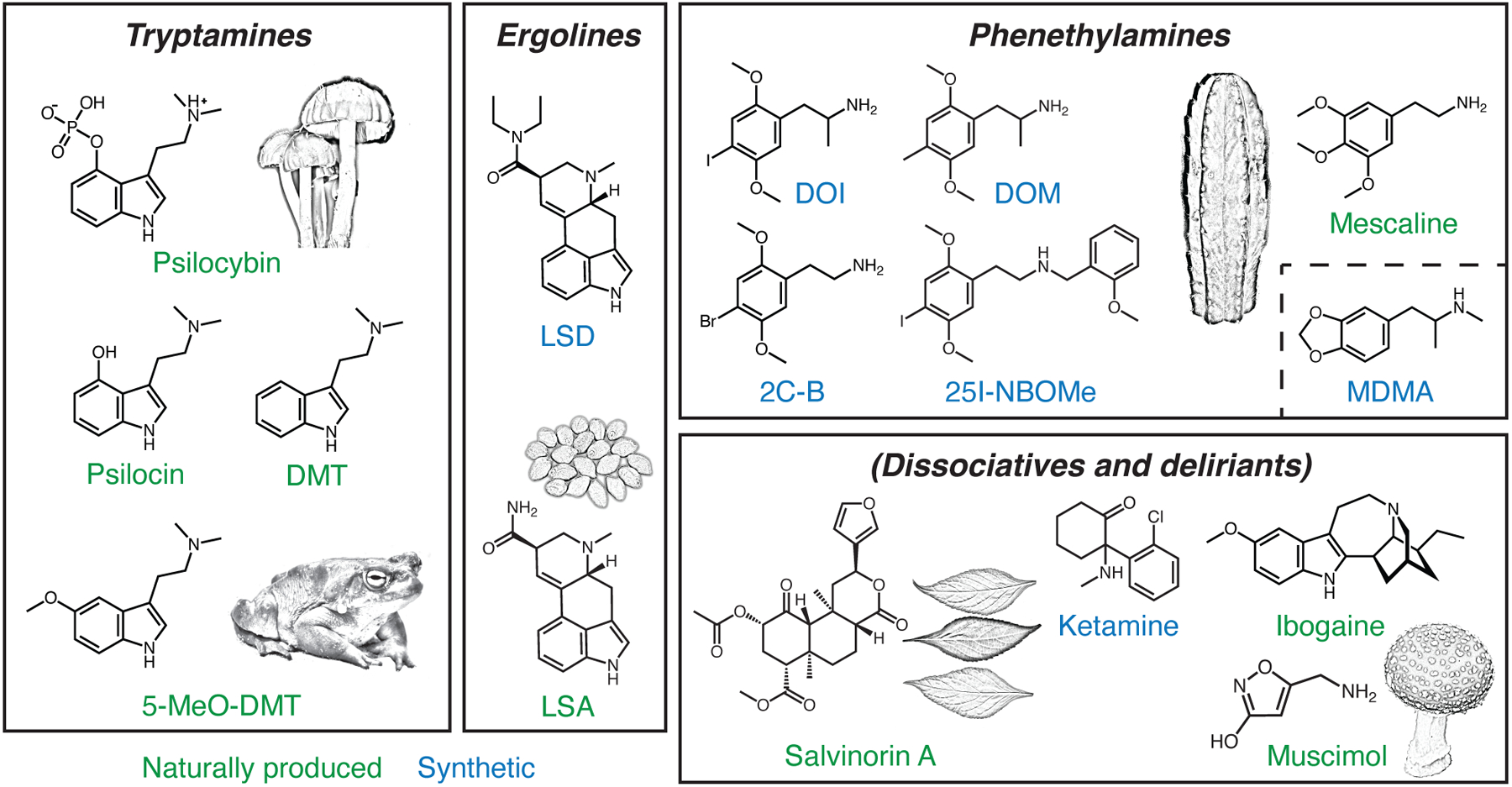
Classic psychedelics can be grouped based on their chemical structures into three major subtypes: tryptamines, ergolines, and phenethylamines. Empathogens such as MDMA are phenethylamines but differ from other compounds in the group by their weak or lack of binding to serotonin receptors. Dissociatives and deliriants are compounds with related psychoactive properties. Some psychedelics can be found in natural sources (green), whereas others can only be synthesized in the lab (blue). The illustrations depict examples of natural sources including psilocybe caerulescens for psilocybin, bufo alvarius for 5-MeO-DMT, ipomoea corymbosa seeds for LSA, echinopsis pachanoi for mescaline, salvia divinorum leaves for salvinorin A, and amanita muscaria for muscimol. DMT, N,N-dimethyltryptamine. 5-MeO-DMT, 5-methoxy-N,N-dimethyltryptamine. LSD, lysergic acid diethylamide. LSA, d-lysergic acid amide. DOI, 2,5-dimethoxy-4-iodoamphetamine. DOM, 2,5-dimethoxy-4-methylamphetamine. 2C-B, 4-bromo-2,5-dimethoxyphenethylamine. MDMA, 3,4-methylenedioxymethamphetamine.
In addition to the classical psychedelics, there are atypical compounds that produce related psychological effects, but do not share the same mechanism of action. These include some phenethylamines such as MDMA, deliriants such as muscimol and scopolamine, and dissociatives such as salvinorin A, ibogaine, nitrous oxide, phencyclidine (PCP), and ketamine. These atypical compounds are sometimes referred to as psychedelics, under a broader definition.
Psychedelics can be found naturally in fungi, plants, and animals. Psilocybin, for instance, is present in a few hundred species of mushrooms, some of which were used for healing and spiritual purposes by the Mayan and Aztec cultures of Mesoamerica. Naturally occurring psychedelics such as mescaline (found in peyote and San Pedro cactus) and DMT (as part of ayahuasca) may have also held cultural significance for early Indigenous peoples of the Americas, although the historical prevalence of these practices is less clear and a matter of debate. Other compounds are synthetic and were discovered as part of pharmaceutical development programs; these include MDMA by Anton Köllisch in 1912 and LSD by Albert Hofmann in 1943 (after initial synthesis in 1938). Today, psychedelics can be produced in multiple ways. For example, psilocybin can be obtained through extraction from mushrooms, enzymatic reactions in a bioreactor with bacteria or yeast, or chemical synthesis.
Properties
Psychedelics undergo various chemical reactions upon entering the body. Some are prodrugs, meaning that the parent compound is inert but is converted to metabolites that can enter the brain to be psychoactive. For example, psilocybin is typically taken through the mouth and dephosphorylated to psilocin in the intestinal lining and liver before entering blood circulation. By contrast, DMT is not bioavailable when orally ingested because it is rapidly eliminated by monoamine oxidase A (MAO-A) in the body. Concurrent use of DMT and an MAO inhibitor greatly increases exposure to the drug, leading to enhanced and prolonged drug effect. Depending on the route of administration, psychedelics are generally considered fast-acting drugs. Psilocin has a half-life of 2.5 hours in blood plasma following oral administration of psilocybin in humans with onset of psychoactive effects beginning 20 – 40 minutes, with peak concentration and effects between 60 – 90 minutes, followed by an approximate 60-minute plateau before decreasing concentration. Within 6 – 8 hours, the subjective drug effects have mostly disappeared. However, psilocybin administered intravenously has a shorter half-life of 30 minutes and a much shorter duration of psychoactive effects of 15 – 30 minutes. Eventually, much of the psilocin is converted further to more soluble metabolites such as glucuronides and excreted in urine.
Psychedelics exert varied effects on perception, cognition, and mood. First-person accounts of the drug-evoked experiences indicate both overlapping and dissimilar features across the many compounds. Controlled studies have been performed for several compounds, including psilocybin, LSD, and MDMA. For example, psilocybin is reported to increase feelings of unity and transcendence of time and space, and to produce perceptual alterations akin to visual hallucinations, illusions, and synesthesia. However, it can also induce anxiety and distressing effects including a dread of ego dissolution, i.e., the loss of one’s sense of self. The acute episode is typically remembered long after the drug has left the system; in one study, most participants ranked it two months later as a significant, personally meaningful experience. There are typically minimal intellectual or memory impairments, and only slight autonomic side effects. Dose and route of administration are important variables contributing to the variance in behavioral effects.
A cornerstone of psychedelic administration is the concept of “set and setting”, which emphasizes the importance of participants’ psychological state (set) and the external environment in which the administration takes place (setting). There is evidence that administration in a safe physical environment with interpersonal support can mitigate negative experiences. With repeated use, people develop diminished response to psychedelics. Indeed, drug tolerance is rapidly induced and there is cross-tolerance across different psychedelics, which may be one reason that dependence and abuse are less common than with many other illicit drugs. Psychedelics are generally considered to be physiologically well tolerated, with relatively low toxicity. Adverse events are rare at psychoactive doses when administered in a controlled, supervised clinical setting.
Animal models
Psychedelics are noted for their ability to alter consciousness. Since the subjective experience cannot be observed externally, other proxy measures are needed to study the impact of psychedelics in animals. One popular assay is the head-twitch response. After the administration of a psychedelic, animals such as mice, rats, and rabbits exhibit rapid and stereotypical head movements that can be recorded with high-speed videography or magnetic sensors. When dozens of psychedelics were compared, their hallucinogenic potency in humans correlate with their capacity to evoke head twitches in mice. Moreover, antagonist drugs that block psychedelic-induced hallucinations also abolish head-twitch responses. For these reasons, notwithstanding a few compounds that are known false positives, head-twitch response is considered a reliable surrogate readout for the hallucinatory experience. Another frequently used assay is drug discrimination. Animals undergo operant training for many weeks to distinguish a psychedelic (e.g., LSD) from saline and report by pressing one of two levers. During testing, a novel compound is presented and its ability to substitute for the psychedelic is evaluated by the lever presses. Drug discrimination can yield precise dose-dependence curves; these are typically assessed with rats, with fewer studies in mice and monkeys.
In addition to these psychedelic-selective assays, an array of general behavioral tests can be used to assess other aspects of drug effects. For example, depressive-like behavior can be studied using tests such as learned helplessness and sucrose preference. Psilocybin, for one, has been shown to ameliorate stress-induced deficits in mice. However, these assays were popularized based on their effectiveness in identifying first and second generation antidepressants. An important question is whether the tests are also suitable for characterizing the unique effects of psychedelics. Another example is the study of pro-social effects of MDMA, which can be modeled by measuring social approach in mice (and octopuses!). Fear extinction, the decrease after repeated exposure in fear associated with a conditioned stimulus, models aspects of exposure therapy for posttraumatic stress disorder and may be useful for evaluating the effects of psychedelics in trauma-related disorders. Overall, findings in animals suggests potentially beneficial actions, but extrapolating such findings to humans is difficult, because each species has its own idiosyncratic behavioral repertoire. Moreover, there are species-specific differences in the blood-brain barrier permeability and pharmacokinetics of drugs and their metabolites.
Effects on the brain
The effects of psychedelics on the brain can be considered at multiple levels: molecular, cellular, circuit, and network (Figure 2). At the molecular level, psychedelics activate serotonin receptors at nanomolar concentrations, particularly the 2A subtype of serotonin receptors (5-HT2A). Binding of tryptamine and ergoline psychedelics to 5-HT2A receptors in the brain is required for the compounds’ consciousness-altering effects. The best evidence for this comes from studies involving the administration of selective 5-HT2A antagonist ketanserin and the partial antagonist risperidone, which block the subjective effects of psychedelics. Other receptor subtypes likely also contribute: most psychedelics bind to 5-HT2C receptors as well, and many tryptamines and ergolines have high affinity for 5-HT1A receptors. Empathogens such as MDMA are exceptions because they act primarily by inhibiting serotonin reuptake. Non-serotonin receptors and multi-receptor heterocomplexes may be involved, but have only been examined in few studies. An exciting recent development is the structural determination of a psychedelic-bound 5-HT2A receptor at near-atomic resolution. This detailed reconstruction of the receptor paves the way for computer simulations to test how thousands of candidate compounds may bind to serotonin receptors, enabling a new era of drug discovery.
Figure 2: Psychedelics act on the brain at multiple levels.
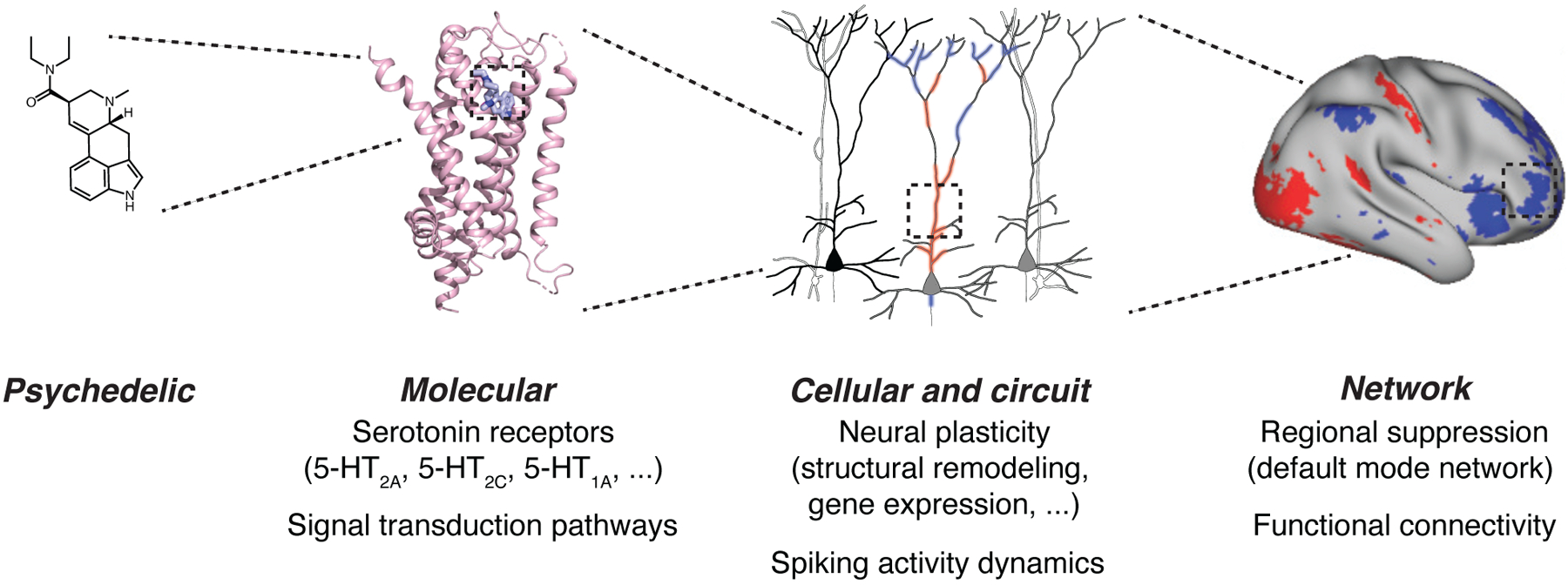
Using LSD as an example, the compound enters the brain and binds to receptors such as the 5-HT2A subtype, activating signal transduction pathways within neurons. The consequences are alterations in gene expression, neural plasticity including remodeling of the synapses and dendrites, and spiking dynamics. At the network level, these effects can be observed as brain-wide changes in regional activation and functional connectivity involving hubs such as the default-mode network. Adapted from Kim et al., Cell, 2020, Savalia et al., Trends Neurosci, 2021, Preller et al., eLife, 2018.
The binding of psychedelics to serotonin receptors activates different signal transduction pathways within neurons. The canonical pathway involves the G-protein Gαq, which upon receptor activation dissociates from the receptor and from its Gβγ partners and activates other downstream effector proteins. There is a parallel, G-protein-independent pathway mediated by β-arrestins. Some psychedelics appear to be biased ligands such that they preferentially engage 5-HT2A receptors in conformations that favor β-arrestin signaling over the G-protein pathway. In contrast to 5-HT2A receptors, the 5-HT1A receptors are Gi/o-protein coupled and activate other signaling proteins.
Engagement of these receptors and signal transduction pathways drives neural plasticity. Psychedelics can alter gene expression, including increasing the transcription of immediate early genes such as c-fos and other activity-dependent transcription factors associated with neural plasticity. Moreover, psychedelics modify the morphology of dendrites, the compartment of a neuron that receives most of the inputs from other cells. In cultured neurons, various psychedelics including LSD, DMT, and DOI, have been shown to induce proliferation of dendritic branches. In live mice, when dendrites are imaged and tracked over time, the administration of a single dose of psilocybin increases the number of dendritic spines, the sites of excitatory inputs, for at least a month. Collectively, these enduring transcriptional and structural effects of psychedelics are important, because they persist beyond the short half-life of psychedelics in the body, reflecting long-lasting modifications to the brain.
Psychedelics are expected to act on certain cell types and in particular brain regions, a selectivity shaped by the complex expression patterns of serotonin receptors. 5-HT2A receptors are found predominantly in the neocortex, thalamus, locus coeruleus, ventral tegmental area, and claustrum. In the neocortex, most 5-HT2A receptors reside on the dendrites of excitatory glutamatergic pyramidal neurons, although some are expressed in other cell types, such as inhibitory GABAergic interneurons. Consistent with this expression pattern, functional studies have shown that the administration of 5-HT agonists to apical dendrites produces an increase in the excitatory postsynaptic potentials. Less is known about the subcellular localization of other serotonin receptor subtypes. The impact of psychedelics on spiking activity dynamics has been reported for a handful of brain regions, such as the frontal cortex and dorsal raphe, but is largely unexplored in other brain areas.
At the level of whole brain, effects of psychedelics on different regions can be visualized using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Studies in humans revealed that psilocybin reduces activities in the medial frontal cortex and posterior cingulate cortex, which compose the default mode network. The default mode network is a set of brain regions that are more active during wakeful rest and less so when engaging the external world. Connectivity analyses of fMRI data suggest that activity covaries more tightly across many regions under the influence of psychedelics. These observations have led to intriguing hypotheses, including the idea that default mode network suppression may explain the experience of ego dissolution. However, many cognitive tasks, disease states, and drugs can also influence the default mode network, so whether this phenomenon is unique to psychedelics remains to be clarified.
Therapeutic potential
Mental health professionals in the 1950s and 1960s studied the potential beneficial effects of psychedelic drugs, especially LSD. While these studies were not up to modern methodological standards, they revealed promising effects in mood disorders and addiction. Unfortunately, unregulated recreational use produced a cultural and legal backlash, culminating in the Controlled Substances Act of 1970, that all but extinguished research into the potential therapeutic benefits of these agents. Research began to pick up in the late 1990s at a handful of institutions in the United States and Europe. It has accelerated enormously over the past ten years, and there is a growing belief among many that psychedelic pharmacology may represent a new era in psychiatric therapeutics.
Most clinical studies described over the past decade have focused on psilocybin. Much attention has been devoted to developing the appropriate set and setting to accompany the dosing experience; this focus sets the therapeutic use of psychedelics apart from many other pharmacological interventions, at least in current research practice. Trust and rapport are developed between the participant and facilitators through conversations prior to drug administration, in which the goal of intentions of treatment are thoroughly discussed. During the dosing session, one or two trained facilitators are present in the room and serve as an anchor for the participant. There are variations: depression studies tend to provide interpersonal support primarily during dosing with potentially a few follow-up integration sessions, but trials for substance use disorders have embedded psychedelic administration in a multi-week course of psychotherapy.
The first controlled studies of psilocybin treatment in the modern era, beginning in 2011, investigated its use to ameliorate the anxiety and depressive symptoms experienced by patients with advanced cancer. Investigators from Johns Hopkins University described a randomized, blinded, crossover study of low versus high dose psilocybin in 51 patients with advanced cancer. The high dose of psilocybin (22 or 30 mg/70 kg) produced a marked and lasting (> 6 months) improvement in anxiety and depression symptoms, quality of life, life meaning, and optimism in 80% of patients. Investigators from New York University simultaneously published a study in 29 cancer sufferers treated with psilocybin (0.3 mg/kg) in comparison to an active placebo, niacin; psilocybin treatment led to lasting improvements. These landmark studies garnered enormous attention and made it clear to the broader community that the modern era of rigorous research into psychedelic therapeutics had arrived.
There have been similarly exciting early studies in the treatment of major depressive disorder (MDD) with psilocybin. Griffiths and colleagues administered two doses of psilocybin (20 mg/70 kg and 30 mg/70 kg, one week apart, in conjunction with psychological support) to 27 patients with MDD, either immediately or after a waiting-list delay. Depression was markedly improved in the psilocybin group, compared to little change in the wait-list group. In another study, Carhart-Harris and colleagues randomized 59 patients with MDD to receive psilocybin (25 mg, with psychological support) and daily placebo to low-dose psilocybin (1 mg, with psychological support) and daily escitalopram, a commonly prescribed antidepressant. Both groups improved; the trend was towards greater improvement in the psilocybin group, though this did not reach statistical significance. These studies are small but impressive and suggest a substantial therapeutic benefit in MDD, comparable to or greater than that of standard medications. Larger studies are needed to corroborate these findings; several are in process.
Treatment studies of psilocybin in numerous other conditions are at an earlier phase. A proof-of-concept study in 10 subjects with alcohol use disorder showed that addition of psilocybin to standard psychotherapeutic treatment improved abstinence for up to 36 weeks. Results from a follow-up, controlled study are expected soon. Studies are underway in obsessive-compulsive disorder, body dysmorphic disorder, anorexia nervosa, headache, cocaine use disorder, smoking, and a variety of other conditions.
In parallel work, investigators led by the Multidisciplinary Association for Psychedelic Studies (MAPS) have investigated the use of MDMA with structured psychotherapy in the treatment of post-traumatic stress disorder (PTSD), with extremely promising results. The 90 participants were randomized to receive either MDMA or placebo, in conjunction with 12 psychotherapy sessions. Those receiving MDMA had a markedly and significantly greater reduction of PTSD symptoms and disability, without significant adverse events. This finding, capping more than two decades of focused work, represents one of the most promising new advances in the treatment of PTSD in many years.
This promising early data, across diagnoses, has caught the attention of regulators. Psilocybin and MDMA remain a Schedule 1 drugs and is thus legally available only in carefully regulated research settings, under federal law. In a few cases, such as Oregon, state and local laws have moved towards a more relaxed stance towards psilocybin use, creating a complicated regulatory landscape. The US Food and Drug Administration granted both psilocybin and MDMA ‘breakthrough therapy’ status for the treatment of depression and PTSD, respectively, acknowledging the promising early data and speeding the regulatory path towards approval, if controlled data continue to be positive. However, the legal status of these drugs has not been changed, and the data are not yet sufficient for them to be approved for any indication.
Open questions
For a more in-depth treatment of the state of the field, we refer the readers to several excellent review articles (e.g., Nichols, Pharmacol. Rev., 2016; Vollenweider and Preller, Nat. Rev. Neurosci. 2020). There are several key questions for which we lack clear answers. We know that the 5-HT2A receptors are essential for the subjective effects of psychedelics. Is it the same or a different receptor subtype that is responsible for the potential therapeutic actions? Relatedly, can we engineer novel psychedelic-like compounds that minimize the subjective effects but retains the beneficial actions? What behavioral assays and neural measurements will be most effective for screening these new compounds? Psychedelics have been shown to promote neural plasticity. Is there something special about those new synaptic connections – for example, do they strengthen certain neural pathways in the brain? Finally, what are the neural and behavioral consequences of microdosing (the use of psychedelics at sub-hallucinogenic dose)?
Summary
We are at an interesting juncture in the research of psychedelics. On the one hand, there is enormous optimism and enthusiasm, motivated by positive results in small, careful studies in mood and anxiety disorders, and early experience in a range of other conditions. On the other hand, it must be acknowledged that work to date remains preliminary and needs validation by robust multi-site studies. Looking ahead, a deeper understanding of the chemistry and neurobiology of psychedelics will facilitate their use and accelerate the discovery of novel compounds – advances that will hopefully fulfill the immense potential of psychedelic pharmacology for treating neuropsychiatric disorders.
Footnotes
Declaration of Interests
BK is in the Scientific Advisory Board for Transcend Therapeutics and Lobe Sciences and has consulted for Ceruvia Lifesciences. CP serves as a consultant for Biohaven, Teva, Lundbeck, Brainsway, Ceruvia Lifesciences, and Freedom Biotech, receives royalties and/or honoraria from Oxford University Press and Elsevier, and has filed a patent on the use of neurofeedback in the treatment of anxiety, which is not relevant to the current work. CP and BK have filed a patent on the use of psilocybin in the treatment of OCD. ACK is in the Scientific Advisory Board for Empyrean Neuroscience.
Further Reading
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, et al. (2012). Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109, 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, Martell J, Blemings A, Erritzoe D, and Nutt DJ (2021). Trial of Psilocybin versus Escitalopram for Depression. N Engl J Med 384, 1402–1411. [DOI] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, and Griffiths RR (2021). Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 78, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck E (2008). Psychedelic Psychiatry: LSD from Clinic to Campus, (Johns Hopkins University Press; ). [Google Scholar]
- Halberstadt AL, Chatha M, Klein AK, Wallach J, and Brandt SD (2020). Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, and Vollenweider FX (2004). Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 172, 145–156. [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, and Griffiths R (2008). Human hallucinogen research: guidelines for safety. J Psychopharmacol 22, 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE, Wacker D, Robertson MJ, Seven AB, Nichols DE, et al. (2020). Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 182, 1574–1588 e1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, et al. (2018). Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CD, and Sanders-Bush E (2002). A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26, 634–642. [DOI] [PubMed] [Google Scholar]
- Nichols DE (2012). Structure-activity relationships of serotonin 5-HT2A agonists. WIREs Membr Transp Signal 1, 559–579. [Google Scholar]
- Nichols DE (2016). Psychedelics. Pharmacol Rev 68, 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalia NK, Shao LX, and Kwan AC (2021). A dendrite-focused framework for understanding the actions of ketamine and psychedelics. Trends Neurosci 44, 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, and Kwan AC (2021). Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544 e2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, and Hell D (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902. [DOI] [PubMed] [Google Scholar]


