Figure 1: Psychedelic compounds.
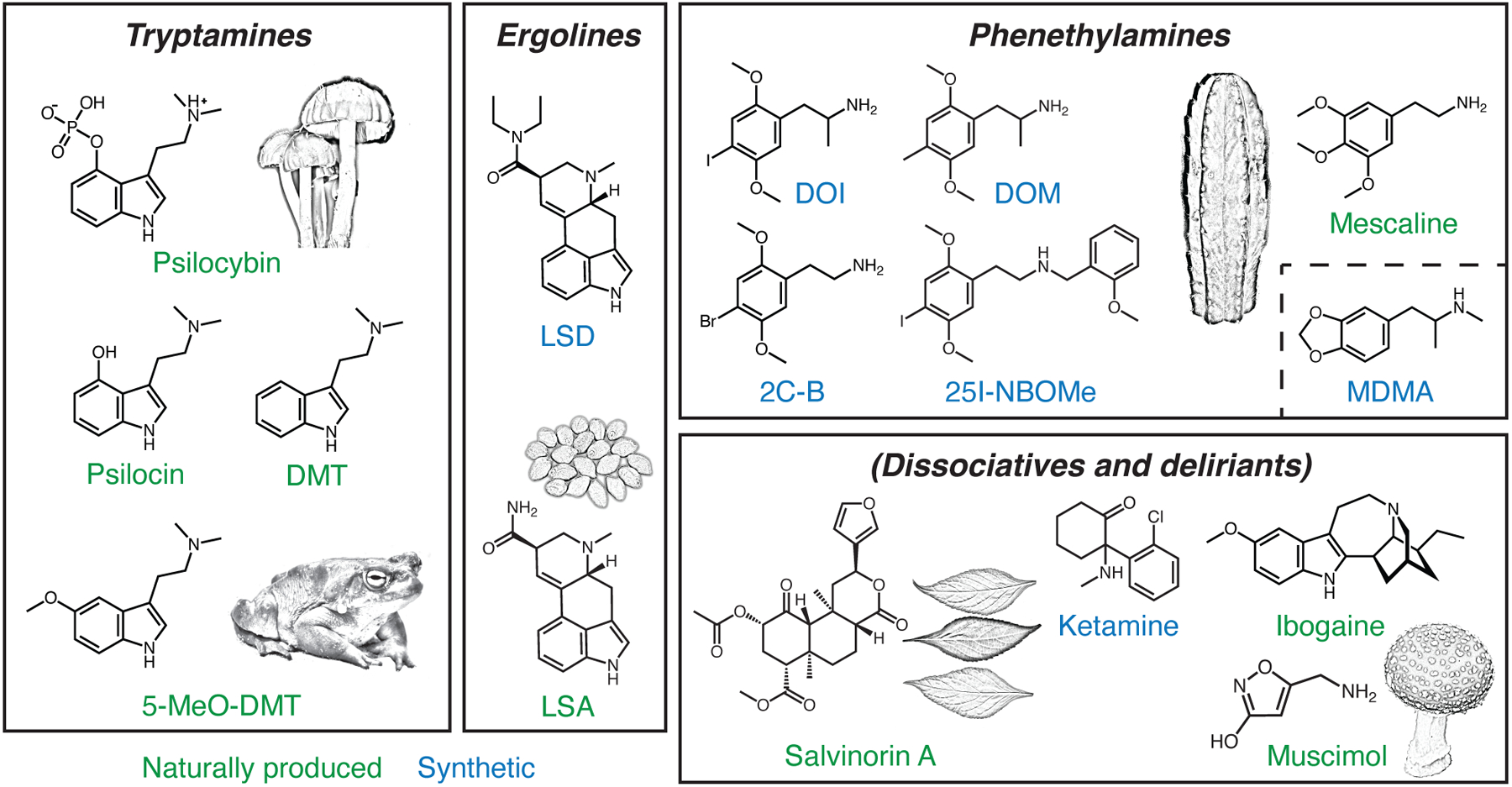
Classic psychedelics can be grouped based on their chemical structures into three major subtypes: tryptamines, ergolines, and phenethylamines. Empathogens such as MDMA are phenethylamines but differ from other compounds in the group by their weak or lack of binding to serotonin receptors. Dissociatives and deliriants are compounds with related psychoactive properties. Some psychedelics can be found in natural sources (green), whereas others can only be synthesized in the lab (blue). The illustrations depict examples of natural sources including psilocybe caerulescens for psilocybin, bufo alvarius for 5-MeO-DMT, ipomoea corymbosa seeds for LSA, echinopsis pachanoi for mescaline, salvia divinorum leaves for salvinorin A, and amanita muscaria for muscimol. DMT, N,N-dimethyltryptamine. 5-MeO-DMT, 5-methoxy-N,N-dimethyltryptamine. LSD, lysergic acid diethylamide. LSA, d-lysergic acid amide. DOI, 2,5-dimethoxy-4-iodoamphetamine. DOM, 2,5-dimethoxy-4-methylamphetamine. 2C-B, 4-bromo-2,5-dimethoxyphenethylamine. MDMA, 3,4-methylenedioxymethamphetamine.
