Abstract
The regeneration of dynamic organs remains challenging because they are intrinsically anisotropic and undergo large volumetric deformation during normal or pathological function. This hampers the durability and applicability of regenerative medicine approaches. To address the challenges of organ dynamics, a new class of patches have emerged with anisotropic and auxetic properties that mimic native tissue biomechanics and accommodate volumetric deformation. Here we outline the critical design, materials, and processing considerations for achieving optimal patch biomechanics according to target pathology and summarize recent advances in biomimetic patches for dynamic organ regeneration. Furthermore, we discuss the challenges and opportunities which, if overcome, would open up new applications in organ regeneration and expedite the clinical translation of patch-based therapeutics.
Keywords: patch, anisotropic, auxetic, regeneration, tissue engineering, negative Poisson’s ratio
Regenerating dynamic organs: the need for anisotropic and auxetic patches
There has been a significant increase in the development and use of therapeutic patches over the last two decades to promote human organ regeneration [1–4]. Early results on their use have been promising across a range of applications including, but not limited to, the treatment of myocardial infarction (MI) [5], cancer [6,7], and chronic wound healing [8]. Dynamic organs (i.e., those that change shape as part of their normal function such as the heart, lung, stomach, and intestines) pose specific challenges when applying patches to their external or internal structures due to their multiscale architectures, constant change in volume (see Glossary), and the anisotropic arrangement of cells and extracellular matrix (ECM) components [9]. Under repeated volumetric dilation and contraction, the surfaces of these organs simultaneously stretch or contract in multiple directions. While there has been tremendous progress in developing patches for tissue regeneration, conventional patches are isotropic and often lack the design features, tensile strength, and elasticity to withstand cyclical loads and accommodate the volumetric expansion of dynamic organs. The resultant biomechanical mismatch between the patch and native organ unduly stresses the organs or leads to premature patch detachment, compromising therapeutic efficacy. To address these dynamics, the research emphasis has shifted over recent years to develop patches that both mimic the biomechanical anisotropy of the organs, and feature auxetic properties that can adapt to volumetric deformation of the organs (Figure 1, Key Figure).
Figure 1. Important considerations for the development of biomimetic patches for the treatment of dynamic organ pathologies.
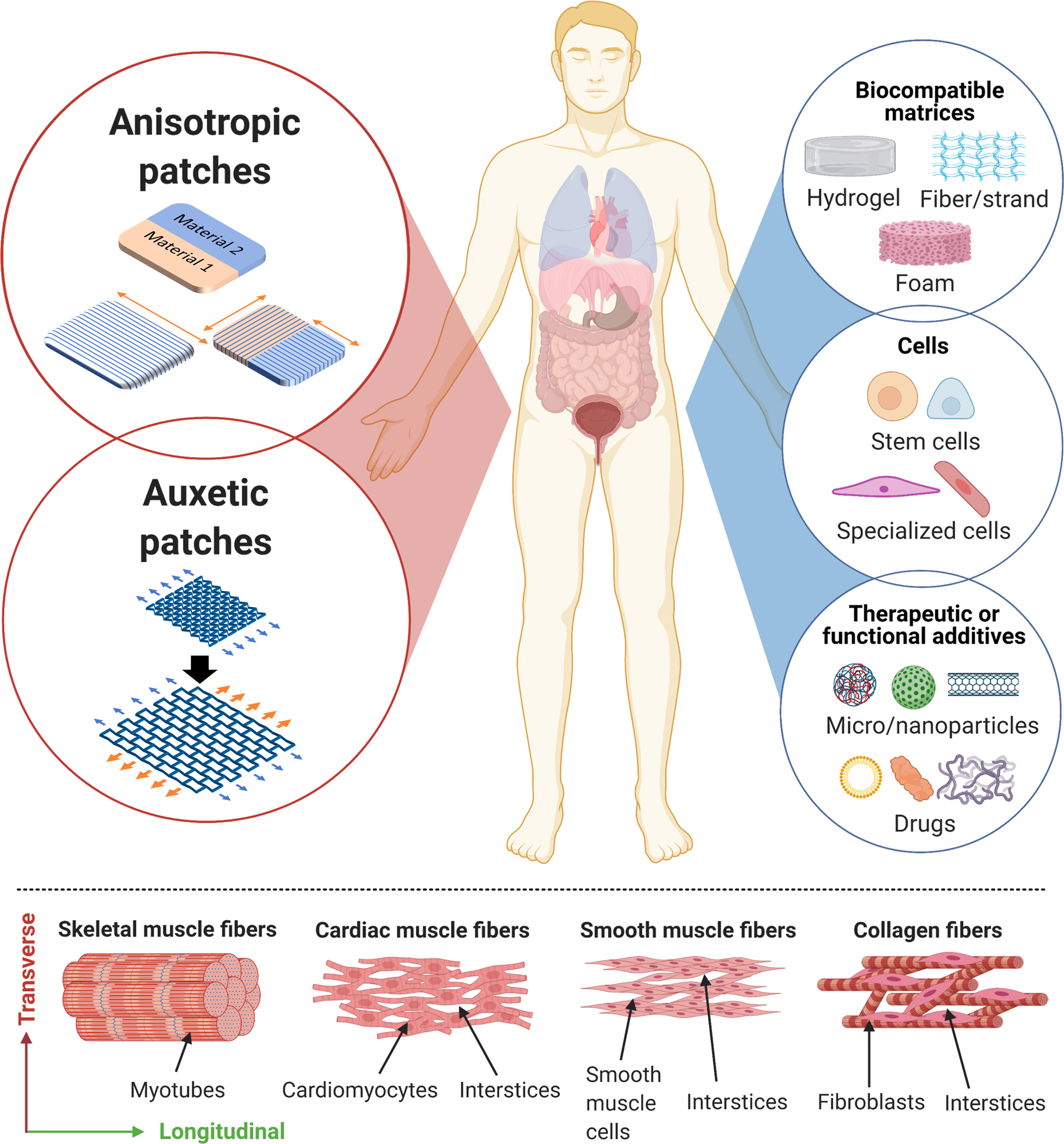
Biomimetic patches can be designed and fabricated with anisotropic and auxetic characteristics (top) to treat different pathologies in dynamic organs. Depending on the target pathology, each patch can be formulated with an appropriate combination of biocompatible materials, cells, and/or therapeutic and functional additives. The microstructure of different tissues is shown in the bottom image. In skeletal muscle, myotubes are closely packed, which only allows for longitudinal or bending deformation. However, the interspersed muscle fibers (in cardiac tissue and smooth muscle of the stomach, intestines, and bladder) and collagen fibers (in the lung and skin) create interstices that allow for simultaneous expansion and contraction of the organ in both the longitudinal and transverse directions. This is also referred to as the auxetic surface characteristic of the organs.
In this review, we highlight a framework for the rational development of biomimetic patches. We first discuss the functional characteristics of different organs that guide decisions on the design, materials, and fabrication method for the patches. We discuss recent advances in biomimetic patch development that aim to recapitulate the anisotropy and auxetic characteristics of dynamic organs. This creates a road map to identify critical biomechanical considerations for patch design catering to the target organ pathology, and develop patches with the desired biomimetic properties (mechanical, biological, chemical, and therapeutic) based on target pathology.
Functional characteristics of dynamic human organs
The design and fabrication of adaptive and biomimicking patches require a thorough understanding of the organ’s functional and deformational characteristics arising from its unique cellular and ECM organization. While every organ features a very nuanced and specific distribution of cell types and ECM, in general terms cardiac tissue contains cardiac muscle fibers formed from cardiomyocytes [10]; the stomach, intestines, and bladder contain fibers formed from smooth muscle cells [11]; and the lung and the skin are largely defined by the organization of collagen fibers produced by fibroblasts [12,13]. Within these organs, the preferential orientation of the cells and ECM components (highlighted as the longitudinal direction in Figure 1 and Figure S1) leads to different properties in different directions or spatial domains, also referred to as intrinsic biomechanical anisotropy [14], which can be spatial or directional (see further details in Figure S1 and Table S1). Spatial anisotropy refers to regional variations in the organ’s constitutive cells and ECM, while directional anisotropy refers to the predominant orientation or patterning of cells and ECM components, such as the muscle and collagen fiber orientation within organs. Due to the inherent complexity of organ composition, organization, and function, every dynamic organ features specific spatial and directional anisotropy, which collectively differentiate the biomechanical properties between different organs and across different regions of an organ.
In addition to the orientation of collagen or muscle fibers, interspersion between the fibers in dynamic organs creates interstitial voids (or interstices; see Figure 1), such as those in cardiac fibers [10], smooth muscle fibers [11], and the collagen fibers of the skin and lung [12,13]. Interstices allow volumetric deformation and simultaneous stretching and contraction across multiple directions at the organ surface. This phenomenon is termed the auxetic property. Except for the diaphragm and some musculoskeletal tissues, interstices in dynamic organs enable the simultaneous propagation of the conduction wave in multiple directions leading to volumetric deformation of these organs. For example, cardiac tissue normally contracts both longitudinally and transversely during systole imparting an auxetic surface deformation [15]. Auxetic structural mechanics are quantified using Poisson’s ratio [16], which lies between −0.8 to −0.6 for cardiac tissue and means that the amount of deformation in the transverse direction is 60%−80% of that in the longitudinal direction [15]. Notably, due to the anisotropic orientation of cardiac muscle fibers, the magnitude of contraction in the longitudinal and transverse directions is different [17,18].
The variation in bulk stiffness, conductivity, volumetric deformation, and strain in different organs is shown in Figure 2. Mechanical stiffness is highest in the skin (>40 MPa [19]) and up to four orders of magnitude lower in lung tissue (<5 kPa [20,21]). Other tissues exhibit stiffnesses between 0.2–20 MPa. Of note, the measured tissue stiffness can vary dramatically depending on the mechanical testing method used and the region being tested [22,23]. For example, tensile testing of bulk skin samples estimates a stiffness between 80–160 MPa [19,22], while indentation tests of the dermis alone estimate a stiffness between 50–100 kPa [24], which is up to three orders of magnitude different. Similarly, the stiffness of bowel tissue measured via indentation testing (2–10 kPa) [25] is several orders of magnitude lower than that determined by tensile testing of bulk tissue (0.8–1.2 MPa) [26]. As such, patches meant to provide structural support need to match the stiffness of the bulk tissue, while patches based on cell attachment and homing will benefit from matching the stiffness of the tissue layer on which the patch is applied [23].
Figure 2. Functional characteristics of dynamic human organs.
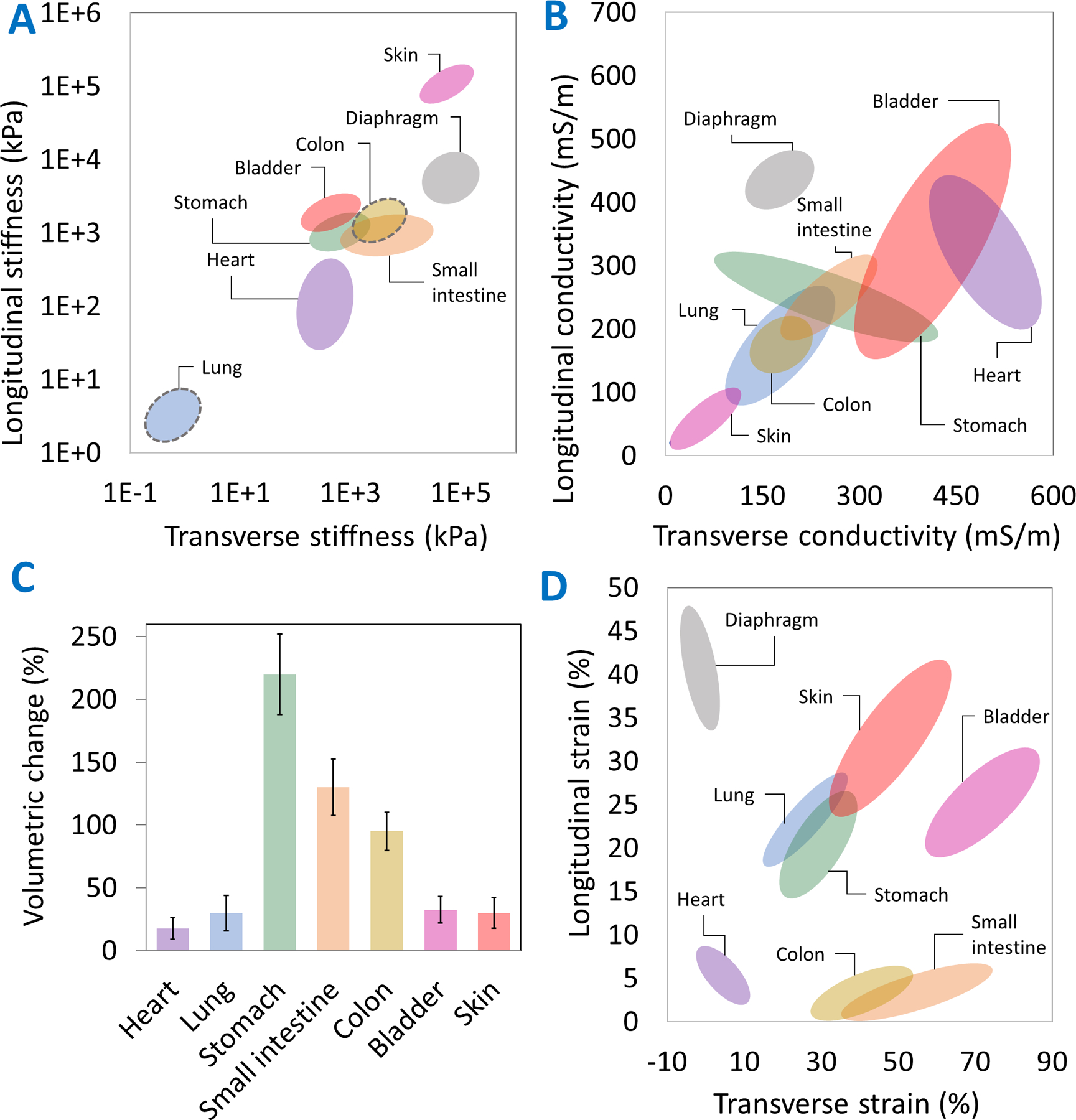
including variations in bulk tissue stiffness (A), conductivity (B), volume (C), and surface strain (D) from the literature (see references in Table S1). For the colon and the lung, there is limited data on the anisotropy of the organ stiffness and, as such, those organs have been marked with a dashed grey circle in (A).
Electrical conductivity for most tissues lies between 50 and 600 mS/m, with cardiac muscle featuring the highest conductivity (300–600 mS/m [27,28]) and the skin the lowest (20–100 mS/m [29,30]). The stomach undergoes the largest volumetric strain (200–280%), followed by the small intestine (100–160% [31]) and the colon (80–120% [32]), with other organs falling in the 20–40% range. The diaphragm is notable for its crucial role in breathing enabled by its very large strain (up to 45% longitudinally [33]) compared to other muscles [33,34]. Amongst other dynamic organs with auxetic surface characteristics, the bladder undergoes some of the highest strains in the transverse direction (up to 80%) during distension [35].
Critical considerations for biomimetic patch development: design, materials, and processes
Design considerations for biomimetic patches
Key design considerations for biomimetic patches featuring anisotropic and auxetic characteristics are highlighted in Figure 3. Spatial anisotropy can be incorporated through zonal variations in the materials, while directional anisotropy can be designed into patches by preferential orientation and patterning of the material itself. To mimic the auxetic characteristics of dynamic organs, patches can feature unique microarchitectures that impart a negative Poisson’s ratio [16,36,37]. Note that some of the auxetic designs such as re-entrant honeycomb and arrowhead are directionally anisotropic and that the patch mechanics can be different in different directions [38]. This intrinsic anisotropy can allow fine-tuning of the design features to match the patch mechanics to that of the native organ [38]. Nevertheless, other auxetic designs can also be made anisotropic by varying the constitutive materials, dimensions, or the type of auxetic design across different directions and spatial domains [39].
Figure 3. Design considerations for engineering biomimetic patches.
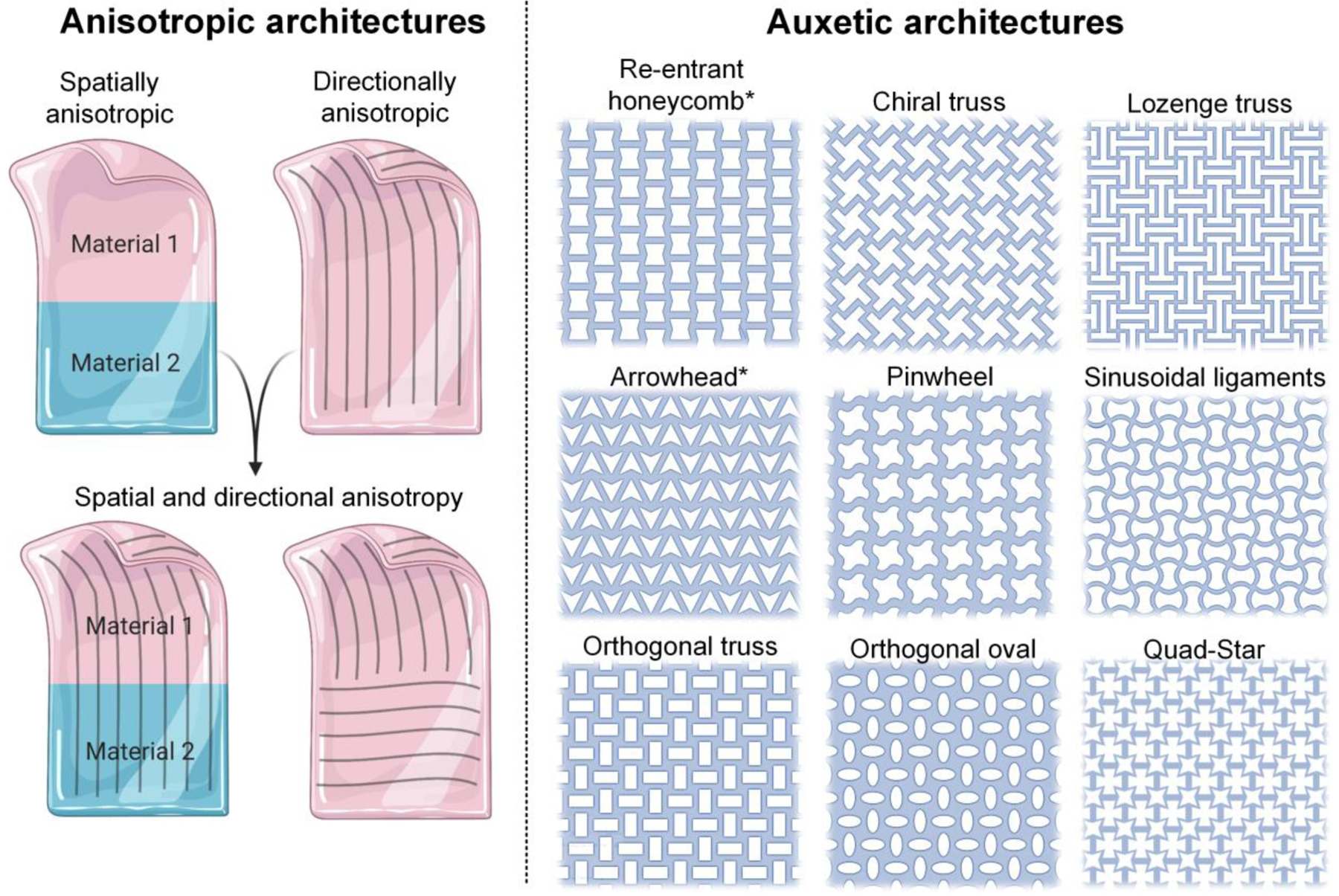
Anisotropic architectures for patches (left) can feature different materials or designs across different spatial domains (spatial anisotropy), direction-specific orientation of the constituent materials (directional anisotropy), or their appropriate combination. The patches can also feature negative Poisson’s ratios through unique auxetic architectures (right), thereby allowing them to expand or contract in multiple directions simultaneously. The re-entrant honeycomb and arrowhead architectures are intrinsically anisotropic, meaning they feature different patch mechanics in the longitudinal or transverse directions. While other auxetic designs are intrinsically isotropic, anisotropy can still be imparted by utilizing different materials or design features across different spatial domains or directions to mimic the biomechanical properties of the native tissue.
Figure360. An author presentation of Figure 3.
Structural materials for patches
The structural materials for patches include biomaterial matrices (highlighted in Table S2) such as hydrogels [5], ECM-mimicking natural fibers [40], synthetic fibers [41], decellularized ECM [42], and foams [43]. A rational selection of the materials, including optimizing the macro-and micro-architecture of the patches, is necessary to match the native organ mechanics. In terms of bulk materials, hydrogel-based patches are more suited to mimic the stiffness of cardiac (100–500 MPa [44,45]) and lung tissues (1.5–5 kPa [20,21]), while thermoplastic or thermosetting-based polymers are suitable for higher stiffness tissues (>500 kPa). However, introducing anisotropic and auxetic arrangements of materials within patches could affect the material properties compared to their bulk counterparts [38,46]. Attributes such as biocompatibility and cell adhesion capability must be considered depending on the target organ pathology and the therapeutic mechanism. Compatibility with fabrication processes including photocrosslinking, printing, or fiber formation (highlighted in Table S2) should also be carefully considered based on the available processes and the desired microarchitectural characteristics.
Living and non-living therapeutic additives for patches
Living or non-living additives such as stem cells or differentiated cells [39], biologics, and micro- or nanoparticles [47] can be added to the patch matrix to further enhance biomimicry and functionality (see Box 1 and Table S3). Cells have been added to patches to accelerate regeneration and integration with the host tissue. For example, directionally anisotropic cardiac patches have been populated with cardiomyocytes to fabricate electroconductive [47] or synchronously beating [48] architectures. Spatially anisotropic skin patches have been populated with multiple cell types (fibroblasts, endothelial cells, neurons, keratinocytes, etc.) corresponding to the hypodermis, dermis and epidermis [49].
Box 1 . Patch modifications to impart biomimicry or therapeutic efficacy.
Approaches that integrate engineering and biological features can further improve the prospects of achieving true biomimicry and optimal patch effectiveness. Integration of tissue-specific cells to populate the patches can allow rapid integration of the patch into tissues and accelerate healing [100]. Critical considerations such as the use of stem cells over differentiated cells [39], or allogeneic vs. autologous cells [100,101] depend upon the target pathology, the treatment mechanism, and the immediacy of treatment. In addition, cell encapsulation within the patches or seeding over patches must still be carefully considered depending on the design, materials, and processing methods. For example, hydrogel-based patches can allow 3D cell encapsulation [70], whereas fibrous patches need cell seeding after the patches have been fabricated [47]. For therapeutic purposes, tailoring the pore size and chemistry renders hydrogels an excellent depot for the delivery and controlled release of therapeutic additives such as small molecules [102], therapeutic proteins [103,104], antibodies [104], toxoids [105], exosomes [106], or drug-loaded microparticles [7] for organ repair. Within fibrous patches, therapeutic additives can be covalently conjugated or incorporated via ionic interactions [107] or encapsulated within polymeric microparticles for controlled delivery [47]. In addition to therapeutic function, additives such as polymeric nanofibers can be used to enhance patch mechanical integrity to improve matrix stiffness [77]. Conductivity can be imparted by coating with polyaniline [38] or polypyrrole [75] or the addition of gold [108] or selenium [109] nanoparticles. Printability can be achieved by introducing silica nanoparticles to improve shear thinning characteristics [110,111]) and the response to external stimuli can be engineered by incorporating gold nanoparticles for photo-thermal response [112]. Notable cells and therapeutic additives, and their use cases for patch fabrication have been highlighted in the Supplementary Information (Table S3).
To create physiologically thick patches with biomimicking cell density and directional anisotropy, cellular reprogramming has been used to produce sufficiently large quantities of cells [50]. Cell sheets have also been used as structural building blocks for biomimetic patches [51,52] as a direct depot of healthy tissue (e.g., muscle [53] or lung epithelium [54]). However, without support matrix, cell sheets lack mechanical integrity [55]. Herein, the patch matrix can not only act as an ideal scaffold and direct cell alignment but can also be used to immunoisolate the encapsulated cells [56].
Furthermore, patches can be designed to incorporate nucleic acids [57], proteins or peptides [58], or vesicles [59,60] to facilitate cellular reprogramming, homing, and differentiation. For example, patches have been modified with barcoded RGD ligands to promote macrophage polarization to prevent inflammatory response [61]. In other studies, vascular endothelial growth factor (VEGF)-loaded anisotropic patches have been used to treat myocardial infarction [47,62], and hydrogel patches with adipose stem cell-derived exosomes or plasma-derived exosomes have been used for wound healing [63] and tendon repair [64], respectively.
Process considerations for biomimetic patches
The selection of appropriate patch fabrication methods capable of processing the materials and achieving the desired architectural characteristics is as important as the design and materials considerations. While a broad range of fabrication processes are available [65,66] (Figure 4 and Table 1), not all are equally suitable for rendering anisotropic and auxetic micro- or nano-structures in the patches. These processes and their hybridization schemes need to be selected based on their materials-process-structure-function interactions, such as the process capabilities (throughput, structural resolution, design freedom, etc.) and the effect of the processing methods on the cells and other therapeutic or functional additives (if any) within the patches [65]. For example, while fiber and fabric formation (FF) processes can allow high-throughput nanoscale feature formation, they are limited in catering to user-defined or patient-specific designs [65]. By contrast, additive manufacturing (AM) processes such as extrusion printing allow user-defined architectures but have limited resolution (>50 µm) and can be time consuming [65]. In contrast, the hybrid process of electrowriting, which combines electrospinning and extrusion printing, can fabricate high-resolution structures and patient-specific geometries [67]. We refer interested readers to other excellent reviews that detail process-materials-structure-function interactions and appropriate selection of the fabrication process [65,66,68].
Figure 4. Illustration of the broad categories of processes that can be used to fabricate biomimetic patches.
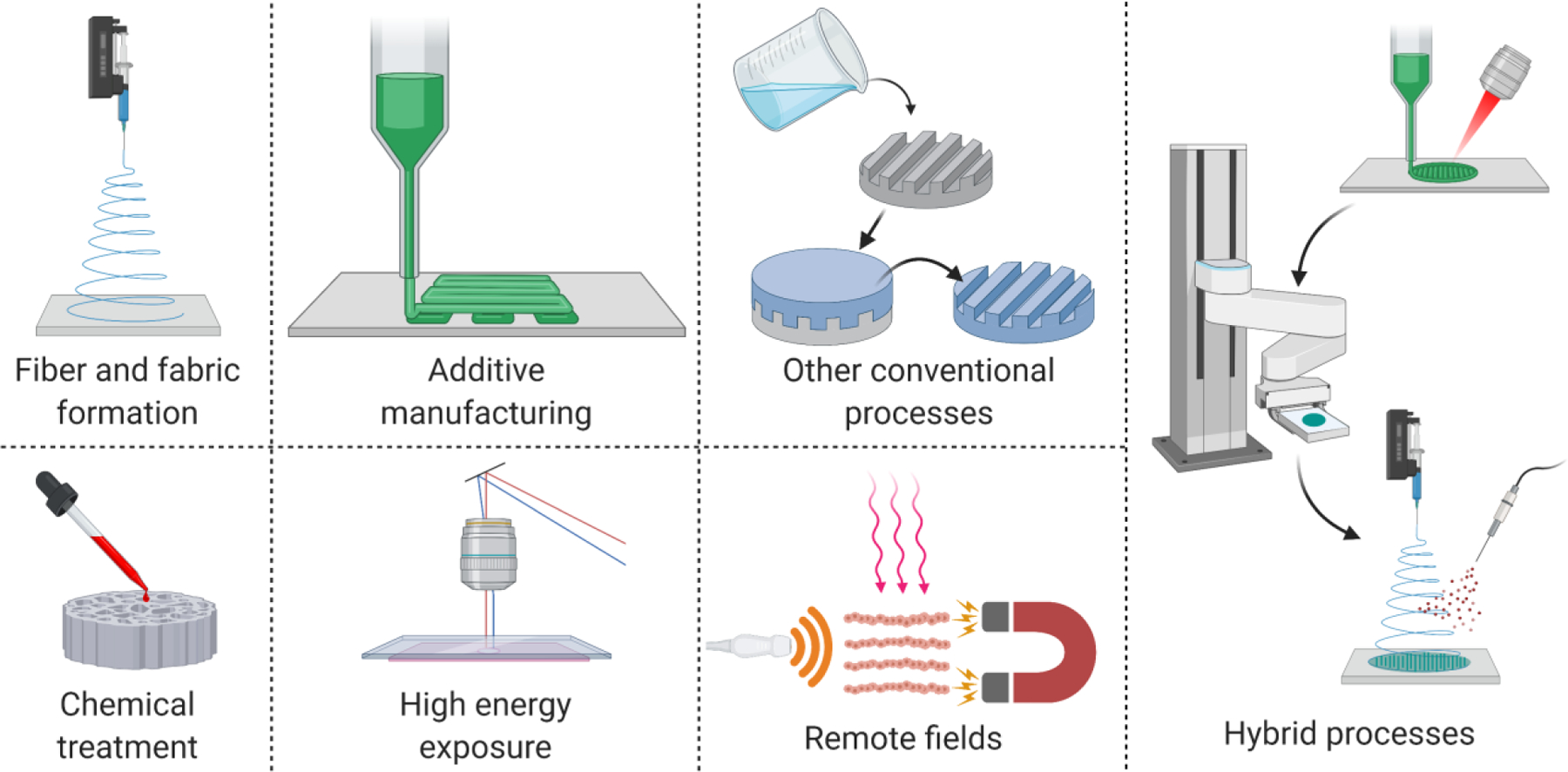
Sub-processes within each category and their typical resolution (values derived from [65]) are listed in Table 1. Fiber and fabric formation (FF) processes involve the use of woven and non-woven methods for fabrication of micro- and nanoscale fibrous architectures [113]. These include electrospinning, melt/solution spinning, blowing, whereas fabric formation processes include knitting, braiding, and weaving [113]. Additive manufacturing (AM) processes involve layer-wise material deposition or curing using extrusion, droplet-deposition, projection lithography or multiphoton lithography [114,115]. High energy exposure (HEE) processes include transferring of high energy particles to cause material ablation and include photolithography and electron beam lithography and laser ablation [116,117]. Chemical treatment (CT) uses methods such as electrochemical etching and modification of patch surfaces via chemical exposure [52]. Remote fields (RF) include processes that rely on acoustic, magnetic, electric, or optical fields to manipulate the biomaterials and additives within polymer precursors [118]. Other conventional (OC) processes include casting/molding and freeze drying, which are ubiquitous in any laboratory setting [119]. Finally, hybrid processes include appropriate combination of individual processes to complement the process capabilities, of which electrowriting or ultrasound-assisted bioprinting (UAB) are notable examples [120–123].
Table 1:
Fabrication processes and their typical resolution ranges.a
| Process | Resolution | Process | Resolution |
|---|---|---|---|
| Fiber and fabric formation | High energy exposure | ||
| Melt electrospinning | ≥ 300 nm | Photolithography | ≥ 1 µm |
| Solution electrospinning | ≥ 100 nm | Electron beam lithography | ≥ 3 nm |
| Melt spinning | ≥ 300 nm | Laser ablation | ≥ 100 nm |
| Solution spinning | ≥ 4 µm | Chemical treatment | |
| Melt and solution blowing | ≥ 500 nm | Chemical etching | ≥ 1 nm |
| Knitting | ≥ 50 µm | Nanocoating | ≥ 1 nm |
| Braiding and Weaving | ≥ 100 µm | Electrospraying | ≥ 10 nm |
| Additive manufacturing | Remote fields | ||
| Extrusion printing | ≥ 100 µm | Electrophoresis | ≥ 1 nm |
| Inkjet printing | ≥ 10 µm | Optical fields | ≥ 10 nm |
| Laser-assisted printing | ≥ 10 µm | Magnetic fields | ≥ 5 nm |
| Stereolithography | ≥ 50 µm | Acoustic fields | ≥ 5 µm |
| Volumetric bioprinting | ≥ 100 µm | Other conventional processes | |
| Multi-photon lithography | ≥ 50 nm | Casting and molding | ≥ 15 µm |
| Acoustophoretic printing | ≥ 10 µm | Solvent casting particulate leaching | ≥ 30 µm |
| Aspiration-assisted printing | ≥ 200 µm | Thermally-induced phase separation | ≥ 50 nm |
Table adapted from [65].
Latest trends in biomimetic anisotropic and auxetic patches for dynamic organs
Using a combination of smart design and appropriate materials and processing methods, biomimetic patches have been developed to allow the material to conform to the mechanics of various dynamic organs and integrate with the host tissue. Here we highlight recent notable examples of patches featuring spatial and/or directional anisotropy, auxetic characteristics, or an appropriate combination of both.
Biomimetic anisotropic patches
To fabricate multi-layered patches mimicking the directional and spatial anisotropy of the human myocardium, Fleischer and colleagues [47] used a hybrid process of electrospinning and laser patterning (Figure 5A). Microgrooves were fabricated within electrospun albumin fiber scaffolds via laser-patterning, allowing neonatal rat cardiomyocyte (NRCM) elongation and anisotropic electrical wave propagation in ex vivo experiments. In another layer, microchannels patterned within the patches followed by seeding with endothelial cells allowed neovascular formation to facilitate nutrient transport to the patch core. Rim and colleagues used photoetching and molding to develop linearly aligned substrates of tyramine-conjugated carboxymethyl cellulose and alginate to culture sheets of vascular smooth muscle cells with aligned cellular morphology and anisotropic mechanical stiffness [69], which were twice as stiff as non-aligned sheets. By stacking different angular orientations of cell sheets, the authors could biomimic native cardiac or arterial tissue architectures. Noor and colleagues utilized concomitant extrusion printing of patient-derived decellularized omentum loaded with induced pluripotent stem cell (iPSC)-derived cardiomyocytes and a sacrificial gelatin bioink containing iPSC-derived endothelial cells [70]. Vessel placement was optimized by modeling the oxygen transfer in silico, such that the entire patch received adequate gas and nutrient exchange to support cell function. The patches were implanted between two layers of SD rat omentum and after a week demonstrated intact vessels and elongated cardiomyocytes with actinin striation. To fully recapitulate microvessel networks, the printed vessel network would require more detailed mapping of the heart in conjunction with high-resolution fabrication (Table 1). Mehrota and colleagues utilized extrusion printing (an AM process) of a blend ink containing non-mulberry silk fibroin, gelatin methacryloyl (GelMA), and polyethylene glycol dimethacrylate (PEGDMA) to develop directionally anisotropic cardiac patches [48]. Post printing, the silk fibroin was enzymatically crosslinked, while GelMA and PEGDMA were photocrosslinked to fabricate a dual-network structure. In vitro culturing of NRCMs demonstrated aligned cardiomyocytes, sarcomere formation and synchronous NRCM beating. Xu and colleagues developed directionally anisotropic patches via thermally-induced phase separation of biodegradable elastomeric polyurethanes containing poly(ethylene glycol), ε-caprolactone and δ-valerolactone [71]. The patches demonstrated higher stiffness in the longitudinal (1.1 MPa) direction compared to the transverse direction (0.9 MPa), similar to native myocardium. When subcutaneously implanted in the backs of Lewis rats over four weeks, the patches demonstrated high cellular infiltration. Yang and colleagues utilized a hybrid strategy of extrusion printing followed by particulate leaching to fabricate cardiac patches featuring crisscrossed filaments and interconnected micropores composed of poly-[glycerol sebacate] (a thermosetting polymer) and polycaprolactone (PCL, a thermoplastic polymer) [72]. The patches demonstrated flexibility and cardiac-mimicking stiffness. In rats with MI, the patches improved cardiac function over four weeks.
Figure 5. Patches featuring anisotropic (panel A) or auxetic architectures (panel B).
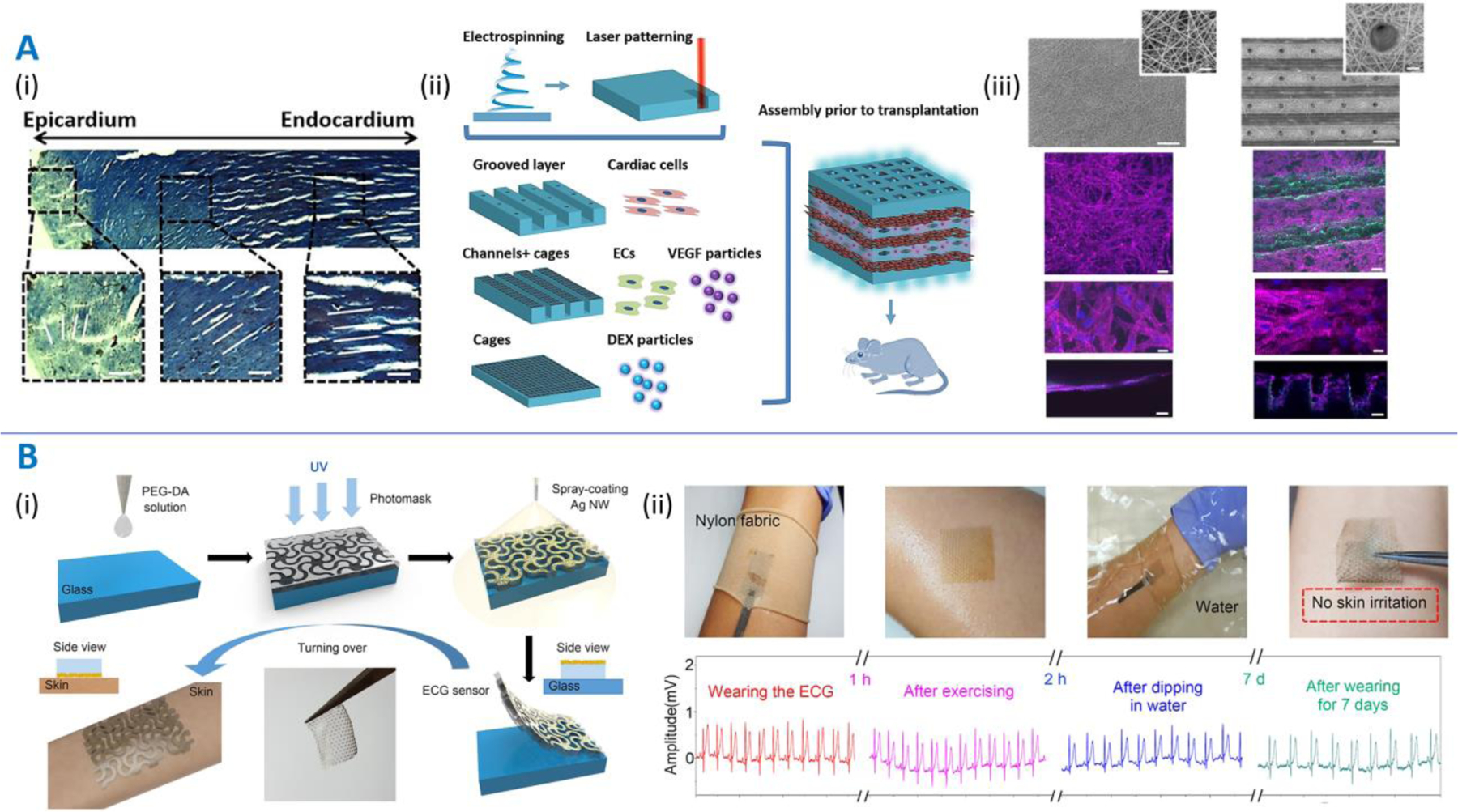
A. Directionally and spatially anisotropic patches. Multi-layered patches mimicking the directional and spatial anisotropy of the human myocardium as demonstrated by Fleischer and colleagues [47]. (i) Masson’s trichrome staining of a transmural section extracted out of the ventricular wall showing variation in fiber orientation in the cardiac tissue. (ii) Scheme of utilizing a bottom-up approach to assembling biomimetic drug (VEGF and dexamethasone)-eluting cardiac patches. (iii) Scanning electron micrographs (top images) demonstrating planar electrospun patches and grooved electrospun patches with micro-holes. Scale bar is 200 µm. Corresponding fluorescence images (bottom) depicting α-sarcomeric actinin (pink) and cell nuclei (blue). Side views are shown in the bottom panels. Scale bars are 50 µm. B. (i) Schematic of the fabrication of auxetic electrode patches developed by Kim and colleagues [73]. PEGDA is dispensed over a glass plate followed by UV crosslinking through a mask and spray coating with Ag nanowires (NWs). (ii) The electrode patches can act as ECG sensors held to the skin via a nylon band, which report changes in ECG measurements depending on the conditions (exercising, in water, etc.) and do not cause any skin irritation over a week. Image reproduced with permission from [47] and [73].
Adaptive and smart patches featuring auxetic architectures
In addition to anisotropic architectures, auxetic architectures that can undergo deformations in multiple directions simultaneously have also been developed. Kim and colleagues [73] utilized casting of PEGDA films followed by UV projection via a photomask to develop patches with serpentine architectures (Figure 5B) similar to the pinwheel architectures (Figure 3). The patches were then spray coated with silver nanowires (AgNWs) to act as an electrocardiogram electrode and a haptic sensor to detect changes in strain. The patches did not irritate human skin and maintained their electrical activity irrespective of the wearing condition and duration. Liu and colleagues used inkjet printing of a photopolymer to create auxetic patches featuring zig-zag microarchitectures (similar to chiral truss) as building blocks [74]. Quantitative modeling under infinitesimal and finite deformations allowed the generation of patch designs based on the desired organ-mimicking Poisson ratios and strain ranges. Xin and colleagues used a similar algorithm for the design optimization of auxetic lattices to achieve lattice stiffnesses and Poisson’s ratios similar to those of different tissues such as the skin, muscle fiber, and lung [46]. Based on a design optimized to match the mechanics of pig-belly skin, light projection 3D printing of a polylactic acid (PLA)‐based shape memory polymer followed by attachment of silver electrodes was used to develop stretchable electroconductive patches, which demonstrated electricity propagation.
Biomimetic patches displaying both anisotropic and auxetic properties
While skeletal muscles and connective tissue pathologies could benefit from anisotropic patches, adding both auxetic characteristics and anisotropy is key to developing patches for other dynamic organs. To this end, Kapnisi and colleagues utilized excimer laser microablation to rapidly fabricate re‐entrant honeycomb auxetic architectures out of an electroconductive chitosan‐polyaniline composite material (Figure 6A) [38]. By changing the design parameters for the re-entrant architectures, they optimized the anisotropic stiffness and conductivity to match that of the myocardium. The auxetic patches conformed better to the cardiac tissue than a non-auxetic patch. In MI-induced rats, the patches adhered to the myocardium for over 14 days, and the MI-patch groups demonstrated significantly lower left ventricular mass compared to the MI-control group, indicating reduced wall stress and attenuated hypertrophy in the MI-patch groups. A longer-term administration over several months (such as the work by Cui and colleagues [39]) may be needed to demonstrate reduction in infarct size and improvement in cardiac function. Olvera and colleagues utilized melt electrowriting (MEW) of PCL to fabricate high-resolution missing-rib auxetic architectures similar in design to the sinusoidal ligaments [75]. The number of sinusoidal loops in the auxetic geometry was altered along the longitudinal and transverse directions to allow recapitulation of cardiac-mimicking anisotropic stiffness [38]. In addition, in situ polymerization of polypyrrole over the patches imparted cardiac-mimicking electroconductivity and demonstrated biocompatibility with seeded cardiac fibroblasts. In vivo evaluation of these patches is now needed to assess their therapeutic effectiveness and biocompatibility. Cui and colleagues developed a physiologically-adaptable cardiac patch via stereolithography printing (AM process) of photocrosslinkable GelMA (5%)-PEGDA(15%) hydrogels [39]. The patches featured wavy patterns to mimic the sinusoidal ligament architectures, wherein the loops in the design could stretch to accommodate volumetric expansion. By altering the fiber diameters (100–400 μm), internal angles (30–60°) between each layer, and the number of layers, patch designs were optimized to mimic the altering fiber orientation from the endocardium to the epicardium and cardiac mechanics in vivo. In vitro cultures under physiologically-relevant mechanical stimulation and in a murine chronic MI model demonstrated improved vascularization and increased cell engraftment within the patches. The approach for the fabrication of the patches has been patented by the authors.
Figure 6. Patches displaying both anisotropic and auxetic properties.
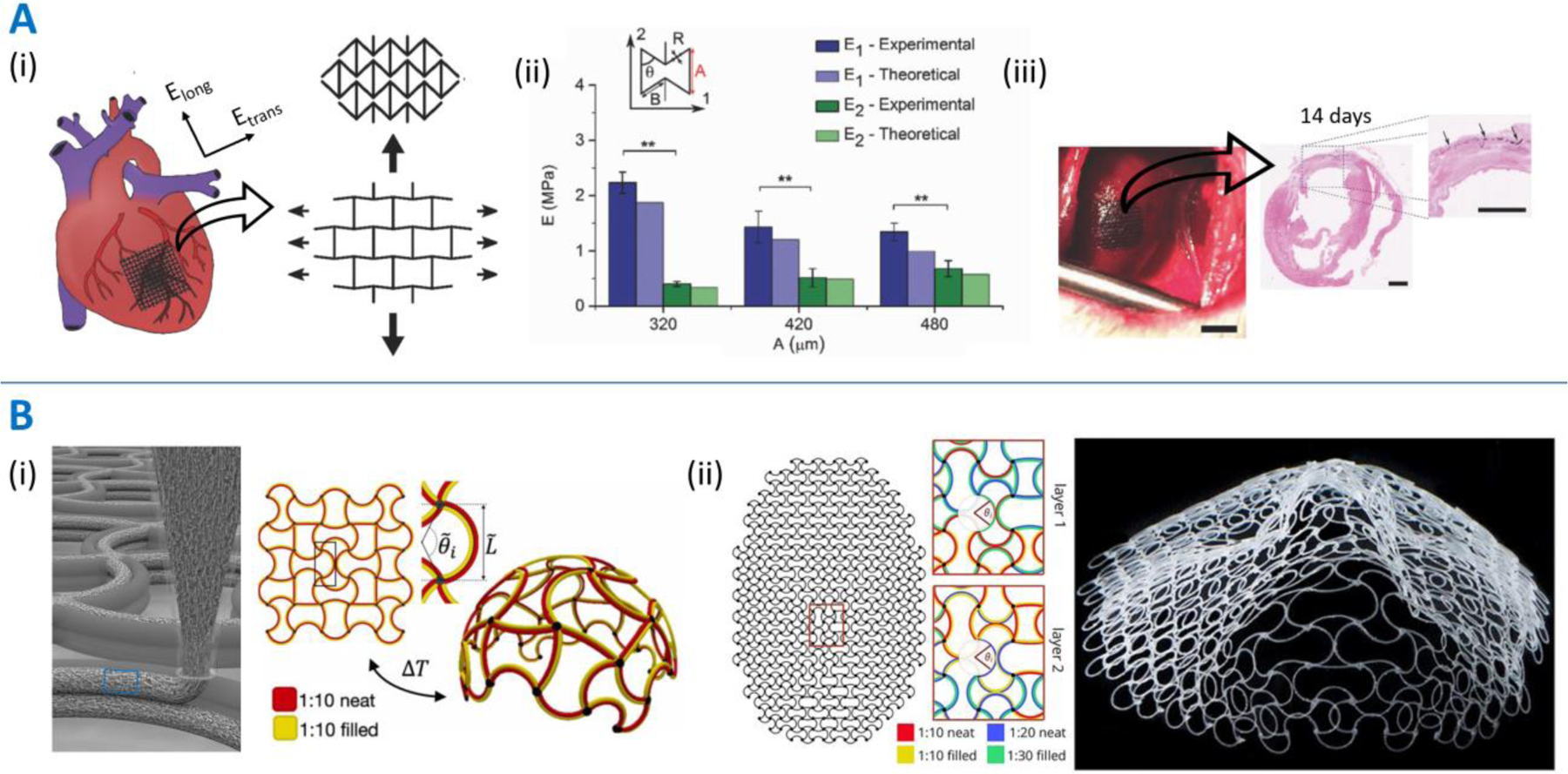
Patches featuring a combination of anisotropy and auxetic architectures developed by Kapnisi and colleagues [38] (Panel A) and Boley and colleagues [77] (Panel B). A. (i) Scheme of administration of the auxetic cardiac patches (AuxCP) on the heart and the deformation characteristics of re-entrant architectures. The stiffer orientation of the re-entrant architecture is aligned with the stiffer transverse direction. (ii) Example of the optimization of design feature – dimension “A” relating to the height of the re-entrant architecture – to achieve cardiac-mimicking longitudinal and transverse stiffness. (iii) AuxCP attached via laser photoadhesion to the left ventricle of a MI-induced rat (scale bar: 4 mm). Hematoxylin and eosin (H&E) stain demonstrating sustained patch adherence (scale bar: 2 mm). B. (i) Schematic of a multi-material printed structure containing PDMS with short glass fibers aligned along the print direction due to shear forces, and schematic of a 2D heterogeneous lattice capable of morphing into a spherical cap when cooled. (ii) Artificial intelligence-based spatial programming of the printing ink constitution, which allows the creation of a 3D face as the resting shape of the flat printed architecture. Image reproduced with permission from [38] and [77].
In addition to optimizing auxetic patch architectures to impart anisotropic properties, using different auxetic designs or dimensions across different spatial domains can further allow the patches to mimic the spatial anisotropy of different dynamic organs. Towards this, Jin and colleagues used melt electro writing (MEW) of PCL to develop heterogeneous scaffolds featuring larger diameter fibers with differing fiber angles to create structural lattices and to allow fine-tuning of the Poisson’s ratio combined with smaller diameter fibers between the larger fibers to allow cellular entrapment, attachment, and elongation [76]. Boley and colleagues developed shape-shifting auxetic (sinusoidal ligament) lattices via extrusion printing of thermo-responsive inks based on a PDMS matrix (Figure 6B) [77]. Different quantities of short glass fibers were used to alter the amount of contraction, while fumed silica nanoparticles were used to impart the shear thinning characteristic essential for extrusion printing. By using different amounts of filler short glass fibers across different regions of the printed architectures, the patch could undergo 4D deformation into spherical shapes or more complex shapes such as a human face. For each of the above studies [76,77], future work is needed to develop the patches for targeted pathologies, and they need to be appropriately tested to assess their biocompatibility and effectiveness.
Concluding remarks and future perspectives
It is evident that adaptive, biomimetic patches have the potential to play a key role in tissue regeneration, in therapeutics, and as sensors. Of the different organ pathologies, biomimetic cardiac patches have been one of the most prolific areas of research [44,78] given the socio-economic and health impacts of cardiovascular diseases [79]. To date, a limited number of patches have been approved for clinical use, such as the ECM-based Cor™ Patch for epicardial tissue repair (https://fda.report/GUDID/00850003965017), or the CardioCel® patch for the repair of cardiac and vascular defects (https://fda.report/GUDID/09348992000358). While these patches help tissue repair they are not curative, in part due to lack of full biomimicry. Most biomimetic cardiac patches still fail to establish full electrical integration with the tissue [80] and provide only limited tissue regeneration [38]. Therefore, there are still critical considerations that must be considered (see Outstanding Questions) to improve patch design, materials, and processing, to create patches that fully integrate with the host tissue and adapt to the demanding mechanics of dynamic organs.
Outstanding questions box.
There have been significant advances in machine learning, artificial intelligence, and computer simulations over the last few years. How can these advances be leveraged to develop predictive algorithms to develop biomimetic architectural structures capable of recapitulating dynamic organs?
To create biomimetic patches, a full understanding of the physiological and bio-mechanical properties of dynamic organs is required. What advances in tissue and organ characterization methods based on imaging and computational modeling are required to accomplish this?
Can biomimetic patches be used for tissue regeneration and complete cure to circumvent the need for organ transplantation?
Minimally invasive surgeries could alleviate risks associated with patch implantation. How can we translate ex vivo printing of auxetic and anisotropic architectures to in vivo printing?
While significant advances have been made towards the fabrication of complex multifunctional cardiac patches, there is an opportunity gap to apply patches to underexplored pathologies (Table S4); for example, the materials or processing of cardiac patches could be appropriately tuned to conform to bladder mechanics and treat different bladder pathologies, such as a drug-eluting patch for bladder cancer [81]. As another example, pneumothorax [82] could be treated with hole filling, auxetic multi-material patches [83] to prevent air leakage.
Fabricating patches fully mimicking dynamic organs requires a better understanding of organ physiology and how clinicopathological characteristics affect the bio-mechanical properties of organs [84,85]. Advances in imaging techniques such as computed tomography [70] and computational models [86] capable of characterizing organ physiology and measuring organ dynamics will be necessary to precisely quantify patient-specific organ macro- and micro-architectures. To precisely tune the auxetic and anisotropic properties of patches, the rational selection of the design and materials can be informed through computational modeling [70] and deep learning [77,87] and use appropriate processes [65] that offer tight control over the resolution. Future research on anisotropic-auxetic architectures will benefit greatly from techniques such as multiphoton lithography, which can fabricate highly complex architectures with nano-scale resolution [88]. Since this process can be very time-consuming, appropriate process hybridization schemes [65] coupled with automation [68] can be exploited to improve throughput, versatility, and repeatability of patch fabrication.
The attachment of patches to internal organs can be quite invasive. Therefore, minimally invasive procedures could significantly enhance patient comfort and translational potential of patches. To this end, in vivo printing of multi-layered anisotropic and auxetic patches loaded with patient-specific specialized cells or stem cells could be used for in situ repair of damaged dynamic organs. Of note, the constant motion and movements of dynamic organs pose additional challenges to in vivo 3D printing and would need to be overcome through technical innovations. Minimally invasive surgery (MIS) is another promising technique to overcome concerns related to invasive surgical procedures. For ease of application, origami-inspired patches have been used to fold over surgical devices [89]; at the application site, surgical forceps can unwind the patch or the patch could self-morph via a memory effect [90]. Notably, patch attachment by suturing could prematurely break patches due to increased stresses at the suture site [38]. Furthermore, sutures are usually placed at the patch vertices [38], which may limit their expansion at the edges and affect mechanical performance. Therefore, patches would benefit from being intrinsically bioadhesive. Bioadhesion can be imparted through chemical modification of the patch materials [91,92], the use of intrinsically bioadhesive materials [5], or bioinspired architectures with proven bioadhesive properties such as microneedles [4,93], tree-frog pads, and micro-suction cups [94].
While the biomechanical or biochemical support provided by patches promotes tissue regeneration [39,95], patches may also need to adapt to the changing properties of the underlying tissue over time. Adaptivity can be imparted by engineering patches with materials [96] that self-degrade over time or upon chemical stimulation [97] such as in the presence of disease-specific markers including reactive oxygen species [98] or matrix metalloproteinases [99]. Of note, to maintain their auxetic structure to conform to the organ mechanics, patches will need to degrade uniformly throughout their geometries.
Finally, patch development must also consider pre-clinical testing requirements in small and large animal models. This will require a priori patch design that matches the species of interest or a redesign to adjust the patch properties, such as stiffness and expandability, to each species. In the latter case (see inter-species comparison of stiffness in Table S5 and Figure S2), extensive retesting will be required if the patch redesign involves changes in the materials, functional additives, and processing conditions.
As the clinical applications of patches for regenerative and diagnostic medicine continue to broaden, the optimization of materials, designs and processes, the standardization of preclinical evaluation models and reliable sources of cells and therapeutic additives, would help to improve practicality and acceptance by end users, which would further accelerate regulatory approval for rapid clinical translation.
Supplementary Material
Highlights.
Dynamic organs (e.g., the heart) combine anisotropic cellular and matrix topology with auxetic surface behavior during volumetric expansion or contraction.
Biomimetic patches with biomechanical anisotropy and auxetic properties would be useful for dynamic organ regeneration to prevent undue stresses on the organ or premature patch detachment and impart biological effects to support organ function.
Biomimetic patches can exert the desired bio/mechanical effects including supporting organ function; cell homing, attachment and alignment; and biomimicking extracellular matrix deposition to aid in organ regeneration.
Design, materials, and processing must all be taken into consideration to achieve optimal patches for dynamic organs according to target pathology and to account for individual variability.
Acknowledgements
We acknowledge funding through the Eshelman Institute for Innovation at the UNC Eshelman School of Pharmacy and the National Institutes of Health (R01CA241679, R01EB023262, and R21GM135853). Figures were created using Biorender.com.
Glossary
- Anisotropy
The characteristic of a structure to exhibit different properties across different directions (directional anisotropy) or spatial regions/zones (spatial anisotropy)
- Auxetic design
Structural design imparting a negative Poisson’s ratio to the structures, i.e., the structures expand laterally when extended and contract laterally when compressed
- % Strain
Change in length relative to the original length = (Lf-Li)*100/Li, where Lf is the final length and Li is the initial length
- % Volumetric change
Change in volume relative to the original volume = (Vf-Vi)*100/Vi, where Vf is the final volume and Vi is the initial volume
- Poisson’s ratio
The negative value of the ratio of the strain across the principal direction (i.e., the direction experiencing higher strain) to the strain in the orthogonal direction. Most common structures contract laterally when expanded, hence exhibiting a positive Poisson’s ratio (usually between 0 and 0.5). In contrast, the surface of most dynamic organs such as the lung or the heart tends to expand or contract in multiple directions simultaneously, thereby exhibiting a negative Poisson’s ratio
- Stiffness
The extent to which an object resists mechanical deformation. It is frequently used interchangeably with the elastic modulus, which is the slope in the linear region of the stress vs. strain curve derived through mechanical testing
- Stress
Force per unit area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lakshmanan R, Krishnan UM, and Sethuraman S (2012). Living cardiac patch: The elixir for cardiac regeneration. Expert Opin. Biol. Ther 12, 1623–1640. Available at: 10.1517/14712598.2012.721770. [DOI] [PubMed] [Google Scholar]
- 2.Bolonduro OA, Duffy BM, Rao AA, Black LD, and Timko BP (2020). From biomimicry to bioelectronics: Smart materials for cardiac tissue engineering. Nano Res 13, 1253–1267. Available at: 10.1007/s12274-020-2682-3. [DOI] [Google Scholar]
- 3.Baik S, Lee HJ, Kim DW, Kim JW, Lee Y, and Pang C (2019). Bioinspired Adhesive Architectures: From Skin Patch to Integrated Bioelectronics. Adv. Mater 31, 1803309. Available at: 10.1002/adma.201803309. [DOI] [PubMed] [Google Scholar]
- 4.Cahill EM, and Ocearbhaill ED (2015). Toward Biofunctional Microneedles for Stimulus Responsive Drug Delivery. Bioconjug. Chem 26, 1289–1296. [DOI] [PubMed] [Google Scholar]
- 5.Lin X, Liu Y, Bai A, Cai H, Bai Y, Jiang W, Yang H, Wang X, Yang L, Sun N, et al. (2019). A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat. Biomed. Eng 3, 632–643. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Ye Y, Hochu GM, Sadeghifar H, and Gu Z (2016). Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett 16, 2334–2340. Available at: https://pubs.acs.org/sharingguidelines. [DOI] [PubMed] [Google Scholar]
- 7.Fenn SL, Charron PN, and Oldinski RA (2017). Anticancer Therapeutic Alginate-Based Tissue Sealants for Lung Repair. ACS Appl. Mater. Interfaces 9, 23409–23419. Available at: www.acsami.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataria K, Gupta A, Rath G, Mathur RB, and Dhakate SR (2014). In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int. J. Pharm 469, 102–110. Available at: 10.1016/j.ijpharm.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GR, and Tojeira A (2013). Role of anisotropy in tissue engineering. In Procedia Engineering (Elsevier Ltd), pp. 117–125. Available at: 10.1016/j.proeng.2013.05.100. [DOI] [Google Scholar]
- 10.Whitaker RH (2010). Anatomy of the heart. Medicine (Baltimore) 38, 333–335. [Google Scholar]
- 11.Hafen BB, and Burns B (2020). Anatomy, Smooth Muscle (StatPearls Publishing) [PubMed]
- 12.Tomoda K, Kimura H, and Osaki S (2013). Distribution of Collagen Fiber Orientation in the Human Lung. Anat. Rec 296, 846–850. Available at: 10.1002/ar.22649. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro JF, dos Anjos EHM, Mello MLS, and de Campos Vidal B (2013). Skin Collagen Fiber Molecular Order: A Pattern of Distributional Fiber Orientation as Assessed by Optical Anisotropy and Image Analysis. PLoS One 8, e54724. Available at: 10.1371/journal.pone.0054724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauck RL, Baker BM, Nerurkar NL, Burdick JA, Li W-J, Tuan RS, and Elliott DM (2009). Engineering on the Straight and Narrow: The Mechanics of Nanofibrous Assemblies for Fiber-Reinforced Tissue Regeneration. Tissue Eng. Part B Rev 15, 171–193. Available at: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraiuoli P, Fixsen LS, Kappler B, Lopata RGP, Fenner JW, and Narracott AJ (2019). Measurement of in vitro cardiac deformation by means of 3D digital image correlation and ultrasound 2D speckle-tracking echocardiography. Med. Eng. Phys 74, 146–152. Available at: 10.1016/j.medengphy.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Mir M, Ali MN, Sami J, and Ansari U (2014). Review of mechanics and applications of auxetic structures. Adv. Mater. Sci. Eng 2014. Available at: 10.1155/2014/753496. [DOI] [Google Scholar]
- 17.D’Angelis CA, Coalson JJ, and Ryan RM (2011). Structure of the Respiratory System: Lower Respiratory Tract. In Pediatric Critical Care (Elsevier Inc.), pp. 490–498. Available at: 10.1016/B978-0-323-07307-3.10036-9. [DOI] [Google Scholar]
- 18.Nasehi Tehrani J, McEwan A, and Wang J (2016). Lung surface deformation prediction from spirometry measurement and chest wall surface motion. Med. Phys 43, 5493–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annaidh AN, Ottenio M, Bruyère K, Destrade M, and Gilchrist MD (2010). Mechanical properties of excised human skin. In IFMBE Proceedings (Springer, Berlin, Heidelberg: ), pp. 1000–1003. Available at: 10.1007/978-3-642-14515-5_255. [DOI] [Google Scholar]
- 20.De Hilster RHJ, Sharma PK, Jonker MR, White ES, Gercama EA, Roobeek M, Timens W, Harmsen MC, Hylkema MN, and Burgess JK (2020). Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am. J. Physiol. - Lung Cell. Mol. Physiol 318, L698–L704. Available at: 10.1152/ajplung.00451.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. (2012). Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med 186, 866–876. Available at: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham HK, McConnell JC, Limbert G, and Sherratt MJ (2019). How stiff is skin? Exp. Dermatol 28, 4–9. Available at: 10.1111/exd.13826. [DOI] [PubMed] [Google Scholar]
- 23.Guimarães CF, Gasperini L, Marques AP, and Reis RL (2020). The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater, 1–20. Available at: 10.1038/s41578-019-0169-1. [DOI] [Google Scholar]
- 24.Pailler-Mattei C, Bec S, and Zahouani H (2008). In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med. Eng. Phys 30, 599–606. Available at: 10.1016/j.medengphy.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Stewart DC, Berrie D, Li J, Liu X, Rickerson C, Mkoji D, Iqbal A, Tan S, Doty AL, Glover SC, et al. (2018). Quantitative assessment of intestinal stiffness and associations with fibrosis in human inflammatory bowel disease. PLoS One 13, 1–16. Available at: 10.1371/journal.pone.0200377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egorov VI, Schastlivtsev IV, Prut EV, Baranov AO, and Turusov RA (2002). Mechanical properties of the human gastrointestinal tract. J. Biomech 35, 1417–1425. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0021929002000842. [DOI] [PubMed] [Google Scholar]
- 27.Stinstra JG, Hopenfeld B, and MacLeod RS (2005). On the passive cardiac conductivity. Ann. Biomed. Eng 33, 1743–1751. Available at: 10.1007/s10439-005-7257-7. [DOI] [PubMed] [Google Scholar]
- 28.Faes TJC, Van Der Meij HA, De Munck JC, and Heethaar RM (1999). The electric resistivity of human tissues (100 HZ-10 MHZ): A meta- analysis of review studies. Physiol. Meas 20. Available at: 10.1088/0967-3334/20/4/201. [DOI] [PubMed] [Google Scholar]
- 29.Gabriel C, Peyman A, and Grant EH (2009). Electrical conductivity of tissue at frequencies below 1 MHz. Phys. Med. Biol 54, 4863–4878. Available at: 10.1088/0031-9155/54/16/002. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel C (1996). Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies Available at: http://www.dtic.mil/docs/citations/ADA303903. [Google Scholar]
- 31.Jacobs SL, Rozenblit A, Ricci Z, Roberts J, Milikow D, Chernyak V, and Wolf E (2007). Small bowel faeces sign in patients without small bowel obstruction. Clin. Radiol 62, 353–357. [DOI] [PubMed] [Google Scholar]
- 32.Turner D, Walsh CM, Benchimol EI, Mann EH, Thomas KE, Chow C, McLemon RA, Walters TD, Swales J, Steinhart AH, et al. (2008). Severe paediatric ulcerative colitis: Incidence, outcomes and optimal timing for second-line therapy. Gut 57, 331–338. [DOI] [PubMed] [Google Scholar]
- 33.Gauthier AP, Verbanck S, Estenne M, Segebarth C, Macklem PT, and Paiva M (1994). Three-dimensional reconstruction of the in vivo human diaphragm shape at different lung volumes. J. Appl. Physiol 76, 495–506. Available at: 10.1152/jappl.1994.76.2.495. [DOI] [PubMed] [Google Scholar]
- 34.Merrell AJ, and Kardon G (2013). Development of the diaphragm - a skeletal muscle essential for mammalian respiration. FEBS J 280, 4026–4035. Available at: 10.1111/febs.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagle AS, Klausner AP, Varghese J, Bernardo RJ, Colhoun AF, Barbee RW, Carucci LR, and Speich JE (2017). Quantification of bladder wall biomechanics during urodynamics: A methodologic investigation using ultrasound. J. Biomech 61, 232–241. Available at: 10.1016/j.jbiomech.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T, Chen Y, Hu X, Li Y, and Wang L (2018). Exploiting negative Poisson’s ratio to design 3D-printed composites with enhanced mechanical properties. Mater. Des 142, 247–258. Available at: 10.1016/j.matdes.2018.01.034. [DOI] [Google Scholar]
- 37.Schwerdtfeger J, Wein F, Leugering G, Singer RF, Körner C, Stingl M, and Schury F (2011). Design of auxetic structures via mathematical optimization. Adv. Mater 23, 2650–2654. Available at: 10.1002/adma.201004090. [DOI] [PubMed] [Google Scholar]
- 38.Kapnisi M, Mansfield C, Marijon C, Guex AG, Perbellini F, Bardi I, Humphrey EJ, Puetzer JL, Mawad D, Koutsogeorgis DC, et al. (2018). Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction. Adv. Funct. Mater 28, 1800618. Available at: 10.1002/adfm.201800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui H, Liu C, Esworthy T, Huang Y, Yu ZX, Zhou X, San H, Lee SJ, Hann SY, Boehm M, et al. (2020). 4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction. Sci. Adv 6, eabb5067. Available at: https://advances.sciencemag.org/content/6/26/eabb5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izadifar M, Chapman D, Babyn P, Chen X, and Kelly ME (2018). UV-Assisted 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering. Tissue Eng. - Part C Methods 24, 74–88. Available at: 10.1089/ten.tec.2017.0346. [DOI] [PubMed] [Google Scholar]
- 41.Yin Y, Mo J, and Feng J (2020). Conductive fabric patch with controllable porous structure and elastic properties for tissue engineering applications. J. Mater. Sci 55, 17120–17133. Available at: 10.1007/s10853-020-05219-9. [DOI] [Google Scholar]
- 42.Shah M, Kc P, and Zhang G (2019). In Vivo Assessment of Decellularized Porcine Myocardial Slice as an Acellular Cardiac Patch. ACS Appl. Mater. Interfaces 11, 23893–23900. Available at: 10.1021/acsami.9b06453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Vlierberghe S, Graulus GJ, Samal SK, Van Nieuwenhove I, and Dubruel P (2014). Porous hydrogel biomedical foam scaffolds for tissue repair. In Biomedical Foams for Tissue Engineering Applications (Elsevier Ltd.), pp. 335–390. Available at: 10.1533/9780857097033.2.335. [DOI] [Google Scholar]
- 44.Silvestri A, Boffito M, Sartori S, and Ciardelli G (2013). Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromol. Biosci 13, 984–1019. [DOI] [PubMed] [Google Scholar]
- 45.Neal RA, Jean A, Park H, Wu PB, Hsiao J, Engelmayr GC, Langer R, and Freed LE (2013). Three-dimensional elastomeric scaffolds designed with cardiac-mimetic structural and mechanical features. Tissue Eng. - Part A 19, 793–807. Available at: 10.1089/ten.tea.2012.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xin X, Liu L, Liu Y, and Leng J (2020). 4D Printing Auxetic Metamaterials with Tunable, Programmable, and Reconfigurable Mechanical Properties. Adv. Funct. Mater 30, 2004226. Available at: 10.1002/adfm.202004226. [DOI] [Google Scholar]
- 47.Fleischer S, Shapira A, Feiner R, and Dvir T (2017). Modular assembly of thick multifunctional cardiac patches. Proc. Natl. Acad. Sci. U. S. A 114, 1898–1903. Available at: 10.1073/pnas.1615728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehrotra S, de Melo BAG, Hirano M, Keung W, Li RA, Mandal BB, and Shin SR (2020). Nonmulberry Silk Based Ink for Fabricating Mechanically Robust Cardiac Patches and Endothelialized Myocardium-on-a-Chip Application. Adv. Funct. Mater 30, 1–16. Available at: 10.1002/adfm.201907436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahmati M, Blaker JJ, Lyngstadaas SP, Mano JF, and Haugen HJ (2020). Designing multigradient biomaterials for skin regeneration. Mater. Today Adv 5, 100051. [Google Scholar]
- 50.Song SY, Kim H, Yoo J, Kwon SP, Park BW, Kim JJ, Ban K, Char K, Park HJ, and Kim BS (2020). Prevascularized, multiple-layered cell sheets of direct cardiac reprogrammed cells for cardiac repair. Biomater. Sci 8, 4508–4520. Available at: https://pubs.rsc.org/en/content/articlehtml/2020/bm/d0bm00701c. [DOI] [PubMed] [Google Scholar]
- 51.Gallego-Perez D, Higuita-Castro N, Sharma S, Reen RK, Palmer AF, Gooch KJ, Lee LJ, Lannutti JJ, and Hansford DJ (2010). High throughput assembly of spatially controlled 3D cell clusters on a micro/nanoplatform. Lab Chip 10, 775–782. Available at: https://pubs.rsc.org/en/content/articlehtml/2010/lc/b919475d. [DOI] [PubMed] [Google Scholar]
- 52.Chen M, Xu Y, Zhang T, Ma Y, Liu J, Yuan B, Chen X, Zhou P, Zhao X, Pang F, et al. (2019). Mesenchymal stem cell sheets: a new cell-based strategy for bone repair and regeneration. Biotechnol. Lett 41, 305–318. Available at: 10.1007/s10529-019-02649-7. [DOI] [PubMed] [Google Scholar]
- 53.Itabashi Y, Miyoshi S, Yuasa S, Fujita J, Shimizu T, Okano T, Fukuda K, and Ogawa S (2005). Analysis of the electrophysiological properties and arrhythmias in directly contacted skeletal and cardiac muscle cell sheets. Cardiovasc. Res 67, 561–570. Available at: www.elsevier.com/locate/cardiores. [DOI] [PubMed] [Google Scholar]
- 54.Nandkumar MA, Yamato M, Kushida A, Konno C, Hirose M, Kikuchi A, and Okano T (2002). Two-dimensional cell sheet manipulation of heterotypically co-cultured lung cells utilizing temperature-responsive culture dishes results in long-term maintenance of differentiated epithelial cell functions. Biomaterials 23, 1121–1130. [DOI] [PubMed] [Google Scholar]
- 55.Haraguchi Y, Shimizu T, Yamato M, and Okano T (2012). Scaffold-free tissue engineering using cell sheet technology. RSC Adv 2, 2184–2190. Available at: www.rsc.org/advances. [Google Scholar]
- 56.Farina M, Alexander JF, Thekkedath U, Ferrari M, and Grattoni A (2019). Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev 139, 92–115. Available at: 10.1016/j.addr.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen J, and Szoka FC (2012). Nucleic acid delivery: The missing pieces of the puzzle? Acc. Chem. Res 45, 1153–1162. Available at: 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muttenthaler M, King GF, Adams DJ, and Alewood PF (2021). Trends in peptide drug discovery. Nat. Rev. Drug Discov 20, 309–325. Available at: www.nature.com/nrd. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson SW, and Nguyen J (2016). Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J. Control. Release 228, 179–190. [DOI] [PubMed] [Google Scholar]
- 60.Wiklander OPB, Brennan M, Lötvall J, Breakefield XO, and Andaloussi SEL (2019). Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med 11, 8521. Available at: http://stm.sciencemag.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Min S, Jeon YS, Choi H, Khatua C, Li N, Bae G, Jung HJ, Kim Y, Hong H, Shin J, et al. (2020). Large and Externally Positioned Ligand-Coated Nanopatches Facilitate the Adhesion-Dependent Regenerative Polarization of Host Macrophages. Nano Lett 20, 7272–7280. Available at: 10.1021/acs.nanolett.0c02655. [DOI] [PubMed] [Google Scholar]
- 62.Rodness J, Mihic A, Miyagi Y, Wu J, Weisel RD, and Li RK (2016). VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater 45, 169–181. [DOI] [PubMed] [Google Scholar]
- 63.Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S, Akbariqomi M, Sanikhani NS, Absalan M, and Tavoosidana G (2020). Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part A 108, 545–556. [DOI] [PubMed] [Google Scholar]
- 64.Shi G, Wang Y, Wang Z, Thoreson AR, Jacobson DS, Amadio PC, Behfar A, Moran SL, and Zhao C (2020). A novel engineered purified exosome product patch for tendon healing: An explant in an ex vivo model. J. Orthop. Res, jor.24859. Available at: 10.1002/jor.24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chansoria P, Schuchard K, and Shirwaiker RA (2020). Process hybridization schemes for multiscale engineered tissue biofabrication. WIREs Nanomedicine and Nanobiotechnology Available at: 10.1002/wnan.1673. [DOI] [PubMed] [Google Scholar]
- 66.Castilho M, de Ruijter M, Beirne S, Villette CC, Ito K, Wallace GG, and Malda J (2020). Multitechnology Biofabrication: A New Approach for the Manufacturing of Functional Tissue Structures? Trends Biotechnol 38, 1316–1328. Available at: 10.1016/j.tibtech.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Dalton PD (2017). Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng 2, 49–57. Available at: 10.1016/j.cobme.2017.05.007. [DOI] [Google Scholar]
- 68.Dalton PD, Woodfield TBF, Mironov V, and Groll J (2020). Advances in Hybrid Fabrication toward Hierarchical Tissue Constructs. Adv. Sci 7, 1902953. Available at: 10.1002/advs.201902953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rim NG, Yih A, Hsi P, Wang Y, Zhang Y, and Wong JY (2018). Micropatterned cell sheets as structural building blocks for biomimetic vascular patches. Biomaterials 181, 126–139. Available at: 10.1016/j.biomaterials.2018.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noor N, Shapira A, Edri R, Gal I, Wertheim L, and Dvir T (2019). 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci 6, 1900344. Available at: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu C, Okpokwasili C, Huang Y, Shi X, Wu J, Liao J, Tang L, and Hong Y (2020). Optimizing Anisotropic Polyurethane Scaffolds to Mechanically Match with Native Myocardium. ACS Biomater. Sci. Eng 6, 2757–2769. Available at: 10.1021/acsbiomaterials.9b01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y, Lei D, Huang S, Yang Q, Song B, Guo Y, Shen A, Yuan Z, Li S, Qing F, et al. (2019). Elastic 3D‐Printed Hybrid Polymeric Scaffold Improves Cardiac Remodeling after Myocardial Infarction. Adv. Healthc. Mater 8, 1900065. Available at: 10.1002/adhm.201900065. [DOI] [PubMed] [Google Scholar]
- 73.Kim HW, Kim TY, Park HK, You I, Kwak J, Kim JC, Hwang H, Kim HS, and Jeong U (2018). Hygroscopic Auxetic On-Skin Sensors for Easy-to-Handle Repeated Daily Use. ACS Appl. Mater. Interfaces 10, 40141–40148. Available at: 10.1021/acsami.8b13857. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, and Zhang Y (2018). Soft network materials with isotropic negative Poisson’s ratios over large strains. Soft Matter 14, 693–703. Available at: https://pubs.rsc.org/en/content/articlehtml/2018/sm/c7sm02052j. [DOI] [PubMed] [Google Scholar]
- 75.Olvera D, Sohrabi Molina M, Hendy G, and Monaghan MG (2020). Electroconductive Melt Electrowritten Patches Matching the Mechanical Anisotropy of Human Myocardium. Adv. Funct. Mater, 1909880. Available at: 10.1002/adfm.201909880. [DOI] [Google Scholar]
- 76.Jin Y, Xie C, Gao Q, Zhou X, Li G, Du J, and He Y (2021). Fabrication of multi-scale and tunable auxetic scaffolds for tissue engineering. Mater. Des 197, 109277. Available at: 10.1016/j.matdes.2020.109277. [DOI] [Google Scholar]
- 77.Boley JW, Van Rees WM, Lissandrello C, Horenstein MN, Truby RL, Kotikian A, Lewis JA, and Mahadevan L (2019). Shape-shifting structured lattices via multimaterial 4D printing. Proc. Natl. Acad. Sci. U. S. A 116, 20856–20862. Available at: 10.1073/pnas.1908806116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Streeter BW, and Davis ME (2019). Therapeutic Cardiac Patches for Repairing the Myocardium. In Advances in Experimental Medicine and Biology (Springer New York LLC; ), pp. 1–24. Available at: 10.1007/5584_2018_309. [DOI] [PubMed] [Google Scholar]
- 79.Rana JS, Khan SS, Lloyd-Jones DM, and Sidney S (2020). Changes in Mortality in Top 10 Causes of Death from 2011 to 2018. J. Gen. Intern. Med, 1–2. Available at: https://wonder.cdc.gov/ucd-icd10.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackman CP, Ganapathi AM, Asfour H, Qian Y, Allen BW, Li Y, and Bursac N (2018). Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159, 48–58. Available at: 10.1016/j.biomaterials.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel TM, Lele SM, et al. (2013). Bladder cancer: Clinical practice guidelines in oncology. JNCCN J. Natl. Compr. Cancer Netw 11, 446–475. Available at: https://jnccn.org/view/journals/jnccn/11/4/article-p446.xml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tenorio LEM, Devine KJ, Lee J, Kowalewski TM, and Barocas VH (2017). Biomechanics of human parietal pleura in uniaxial extension. J. Mech. Behav. Biomed. Mater 75, 330–335. Available at: 10.1016/j.jmbbm.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 83.Ge Q, Chen Z, Cheng J, Zhang B, Zhang Y-F, Li H, He X, Yuan C, Liu J, Magdassi S, et al. (2021). 3D printing of highly stretchable hydrogel with diverse UV curable polymers. Sci. Adv 7, eaba4261. Available at: 10.1126/sciadv.aba4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sin DD, Wu LL, and Man SFP (2005). The relationship between reduced lung function and cardiovascular mortality: A population-based study and a systematic review of the literature. Chest 127, 1952–1959. Available at: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 85.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, and Heymsfield SB (1997). Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J. Appl. Physiol 83, 229–239. Available at: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 86.Lim B, Kim J, Hwang M, Song JS, Lee JK, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, et al. (2020). In situ procedure for high-efficiency computational modeling of atrial fibrillation reflecting personal anatomy, fiber orientation, fibrosis, and electrophysiology. Sci. Rep 10, 1–10. Available at: 10.1038/s41598-020-59372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilt JK, Yang C, and Gu GX (2020). Accelerating Auxetic Metamaterial Design with Deep Learning. Adv. Eng. Mater 22, 1901266. Available at: 10.1002/adem.201901266. [DOI] [Google Scholar]
- 88.Flamourakis G, Spanos I, Vangelatos Z, Manganas P, Papadimitriou L, Grigoropoulos C, Ranella A, and Farsari M (2020). Laser‐made 3D Auxetic Metamaterial Scaffolds for Tissue Engineering Applications. Macromol. Mater. Eng 305, 2000238. Available at: 10.1002/mame.202000238. [DOI] [Google Scholar]
- 89.Wu SJ, Yuk H, Wu J, Nabzdyk CS, and Zhao X (2021). A Multifunctional Origami Patch for Minimally Invasive Tissue Sealing. Adv. Mater 33, 2007667. Available at: 10.1002/adma.202007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, et al. (2017). Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater 16, 1038–1046. [DOI] [PubMed] [Google Scholar]
- 91.Liang S, Zhang Y, Wang H, Xu Z, Chen J, Bao R, Tan B, Cui Y, Fan G, Wang W, et al. (2018). Paintable and Rapidly Bondable Conductive Hydrogels as Therapeutic Cardiac Patches. Adv. Mater 30, 1704235. Available at: 10.1002/adma.201704235. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Shang L, Chen G, Sun L, Zhang X, and Zhao Y (2020). Bioinspired structural color patch with anisotropic surface adhesion. Sci. Adv 6, eaax8258. Available at: https://advances.sciencemag.org/content/6/4/eaax8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang J, Wang J, Huang K, Ye Y, Su T, Qiao L, Hensley MT, Caranasos TG, Zhang J, Gu Z, et al. (2018). Cardiac cell–integrated microneedle patch for treating myocardial infarction. Sci. Adv 4, eaat9365. Available at: https://advances.sciencemag.org/content/4/11/eaat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim DW, Baik S, Min H, Chun S, Lee HJ, Kim KH, Lee JY, and Pang C (2019). Highly Permeable Skin Patch with Conductive Hierarchical Architectures Inspired by Amphibians and Octopi for Omnidirectionally Enhanced Wet Adhesion. Adv. Funct. Mater 29, 1807614. Available at: 10.1002/adfm.201807614. [DOI] [Google Scholar]
- 95.Estrada AC, Yoshida K, Clarke SA, and Holmes JW (2020). Longitudinal Reinforcement of Acute Myocardial Infarcts Improves Function by Transmurally Redistributing Stretch and Stress. J. Biomech. Eng 142, 0210091. Available at: 10.1115/1.4044030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dolan EB, Hofmann B, de Vaal MH, Bellavia G, Straino S, Kovarova L, Pravda M, Velebny V, Daro D, Braun N, et al. (2019). A bioresorbable biomaterial carrier and passive stabilization device to improve heart function post-myocardial infarction. Mater. Sci. Eng. C 103, 109751. Available at: 10.1016/j.msec.2019.109751. [DOI] [PubMed] [Google Scholar]
- 97.Lu Y, Aimetti AA, Langer R, and Gu Z (2016). Bioresponsive materials. Nat. Rev. Mater 2, 1–17. Available at: https://www.nature.com/articles/natrevmats201675. [Google Scholar]
- 98.Yao Y, Ding J, Wang Z, Zhang H, Xie J, Wang Y, Hong L, Mao Z, Gao J, and Gao C (2020). ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials 232, 119726. Available at: 10.1016/j.biomaterials.2019.119726. [DOI] [PubMed] [Google Scholar]
- 99.Hanjaya-Putra D, Wong KT, Hirotsu K, Khetan S, Burdick JA, and Gerecht S (2012). Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels. Biomaterials 33, 6123–6131. Available at: https://dx.doi.org/10.1016%2Fj.biomaterials.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klama-Baryła A, Kitala D, Łabuś W, Kraut M, Glik J, Nowak M, and Kawecki M (2018). Autologous and Allogeneic Skin Cell Grafts in the Treatment of Severely Burned Patients: Retrospective Clinical Study. Transplant. Proc 50, 2179–2187. [DOI] [PubMed] [Google Scholar]
- 101.Jansen Of Lorkeers SJ, Eding JEC, Vesterinen HM, Van Der Spoel TIG, Sena ES, Duckers HJ, Doevendans PA, Macleod MR, and Chamuleau SAJ (2015). Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circ. Res 116, 80–86. Available at: http://circres.ahajournals.org. [DOI] [PubMed] [Google Scholar]
- 102.Kim I, Han EH, Bang W-Y, Ryu J, Min J-Y, Nam HC, Park WH, Chung Y-H, and Lee E (2018). Supramolecular Carbon Monoxide-Releasing Peptide Hydrogel Patch. Adv. Funct. Mater 28, 1803051. Available at: 10.1002/adfm.201803051. [DOI] [Google Scholar]
- 103.Matsuo K, Ishii Y, Kawai Y, Saiba Y, Quan Y-S, Kamiyama F, Hirobe S, Okada N, and Nakagawa S (2013). Analysis of Transcutaneous Antigenic Protein Delivery by a Hydrogel Patch Formulation. J. Pharm. Sci 102, 1936–1947. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0022354915310819. [DOI] [PubMed] [Google Scholar]
- 104.Guziewicz N, Best A, Perez-Ramirez B, and Kaplan DL (2011). Lyophilized silk fibroin hydrogels for the sustained local delivery of therapeutic monoclonal antibodies. Biomaterials 32, 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirobe S, Matsuo K, Quan YS, Kamiyama F, Morito H, Asada H, Takaya Y, Mukai Y, Okada N, and Nakagawa S (2012). Clinical study of transcutaneous vaccination using a hydrogel patch for tetanus and diphtheria. Vaccine 30, 1847–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, and Zhu L (2017). Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9, 4430–4438. Available at: https://pubs.rsc.org/en/content/articlehtml/2017/nr/c7nr00352h. [DOI] [PubMed] [Google Scholar]
- 107.Ding Y, Li W, Zhang F, Liu Z, Zanjanizadeh Ezazi N, Liu D, and Santos HA (2019). Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater 29. Available at: 10.1002/adfm.201802852. [DOI] [Google Scholar]
- 108.Shevach M, Fleischer S, Shapira A, and Dvir T (2014). Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett 14, 5792–5796. Available at: https://pubs.acs.org/sharingguidelines. [DOI] [PubMed] [Google Scholar]
- 109.Kalishwaralal K, Jeyabharathi S, Sundar K, Selvamani S, Prasanna M, and Muthukumaran A (2018). A novel biocompatible chitosan–Selenium nanoparticles (SeNPs) film with electrical conductivity for cardiac tissue engineering application. Mater. Sci. Eng. C 92, 151–160. [DOI] [PubMed] [Google Scholar]
- 110.Peak CW, Singh KA, Adlouni M, Chen J, and Gaharwar AK (2019). Printing Therapeutic Proteins in 3D using Nanoengineered Bioink to Control and Direct Cell Migration. Adv. Healthc. Mater 8, 1801553. Available at: 10.1002/adhm.201801553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chimene D, Kaunas R, and Gaharwar AK (2019). Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater, 1902026. Available at: 10.1002/adma.201902026. [DOI] [PubMed] [Google Scholar]
- 112.Borzenkov M, Chirico G, Pallavicini P, Sperandeo P, Polissi A, Dacarro G, Doveri L, Collini M, Sironi L, Bouzin M, et al. (2020). Nanocomposite Sprayed Films with Photo-Thermal Properties for Remote Bacteria Eradication. Nanomaterials 10, 786. Available at: https://www.mdpi.com/2079-4991/10/4/786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nayak R, Padhye R, Kyratzis IL, Truong YB, and Arnold L (2012). Recent advances in nanofibre fabrication techniques. Text. Res. J 82, 129–147. Available at: https://doi.org/10.1177%2F0040517511424524. [Google Scholar]
- 114.Zadpoor AA, and Malda J (2017). Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng 45, 1–11. [DOI] [PubMed] [Google Scholar]
- 115.Starly B, and Shirwaiker RA (2015). Three-dimensional Bioprinting. In 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine, Zhang L, Fisher JP, and Leong K, eds. (Waltham: Academic Press; ), pp. 57–77. Available at: 10.1016/B978-0-12-800547-7.00003-5. [DOI] [Google Scholar]
- 116.Melchels FPW, Domingos MAN, Klein TJ, Malda J, Bartolo PJ, and Hutmacher DW (2012). Additive manufacturing of tissues and organs. Prog. Polym. Sci 37, 1079–1104. Available at: 10.1016/j.progpolymsci.2011.11.007. [DOI] [Google Scholar]
- 117.Ravi‐Kumar S, Lies B, Zhang X, Lyu H, and Qin H (2019). Laser ablation of polymers: a review. Polym. Int 68, 1391–1401. Available at: 10.1002/pi.5834. [DOI] [Google Scholar]
- 118.Rashidi H, Yang J, and Shakesheff KM (2014). Surface engineering of synthetic polymer materials for tissue engineering and regenerative medicine applications. Biomater. Sci 2, 1318–1331. Available at: 10.1039/C3BM60330J. [DOI] [PubMed] [Google Scholar]
- 119.Armstrong JPK, and Stevens MM (2020). Using Remote Fields for Complex Tissue Engineering. Trends Biotechnol 38, 254–263. Available at: 10.1016/j.tibtech.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dalton PD (2017). Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng 2, 49–57. Available at: 10.1016/j.cobme.2017.05.007. [DOI] [Google Scholar]
- 121.Chansoria P, and Shirwaiker R (2020). 3D bioprinting of anisotropic engineered tissue constructs with ultrasonically induced cell patterning. Addit. Manuf 32, 101042. Available at: 10.1016/j.addma.2020.101042. [DOI] [Google Scholar]
- 122.Chansoria P, and Shirwaiker R (2019). Characterizing the Process Physics of Ultrasound-Assisted Bioprinting. Sci. Rep 9, 13889. Available at: 10.1038/s41598-019-50449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chansoria P, Narayanan LK, Schuchard K, and Shirwaiker RA (2019). Ultrasound-assisted biofabrication and bioprinting of preferentially aligned three-dimensional cellular constructs. Biofabrication 11, 14861. Available at: 10.1088/1758-5090/ab15cf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


