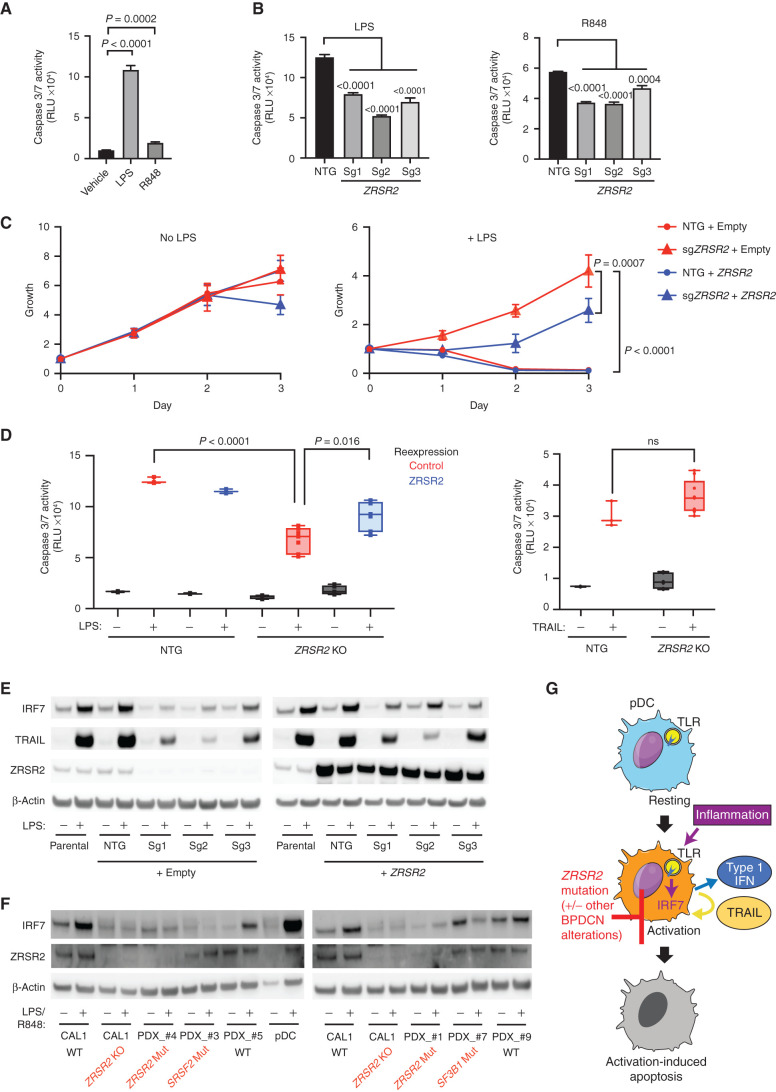
Figure 6.
ZRSR2 mutation impairs pDC apoptosis following TLR stimulation, associated with blunted IRF7 and TRAIL induction. A, Caspase 3/7 activity in parental CAL1 cells after treatment with LPS or R848 compared with vehicle control. B, Caspase 3/7 activity in control or ZRSR2 knockout cells after stimulation with LPS or R848 compared with control sgRNA-expressing cells. C, Relative growth of control and ZRSR2 knockout cells, with wild-type ZRSR2 reexpression or empty vector control, is shown in normal medium (left) or in medium containing LPS (right). D, Caspase 3/7 activity in control and ZRSR2 knockout (KO) cells, with wild-type ZRSR2 reexpression or empty vector control, is shown after treatment with LPS or TRAIL. In A to D, n = 3 biologically independent replicates, groups compared by t test. E, Western blot for IRF7, TRAIL, ZRSR2, and β-actin in parental, nontargeting control, and ZRSR2 knockout CAL1 cells, with or without ZRSR2 reexpression, 24 hours after stimulation with LPS or vehicle. F, Western blot for IRF7, ZRSR2, and β-actin in wild-type or ZRSR2 knockout CAL1 cells, BPDCN PDXs of the indicated genotypes, or normal pDCs, with and without LPS (CAL1) or R848 (BPDCN and normal pDCs) treatment. G, Model for BPDCN-associated mutations' contribution to disease pathogenesis. Normal pDCs respond to inflammation via TLR signaling to IRF proteins, such as IRF7, which causes production of inflammatory mediators, such as type 1 interferons and TRAIL, and promotion of a feedback loop that leads to activation-induced apoptosis. pDCs with ZRSR2 mutations and likely also in the presence of other BPDCN-associated alterations are relatively protected from activation-induced apoptosis because they have impaired upregulation of IRF7 and downstream inflammatory mediators.