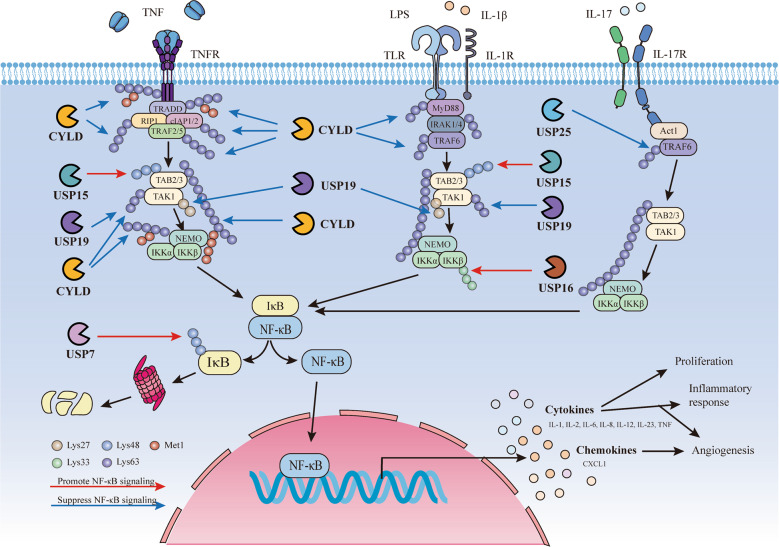
Fig. 2. USPs and NF-κB signalling.
In response to TNF, TNFR conjugates with TRADD, recruiting the kinase RIPK1 and ubiquitin E3 ligase TRAF2/5. TRAF2 associates with cIAP1 and 2 to modify multiple components in TNFR1 complex with Lys63 polyubiquitin (left side of the figure). Stimulation of Toll-like receptors (TLR) or interleukin-1 receptor (IL-1R) induces the arrangement of the Myddosome complex consisting of MyD88, IRAK4, IRAK1. Subsequently, IRAK1 is phosphorylated by IRAK4 and subsequently recruits the ubiquitin E3 ligase TRAF6 (middle side of the figure). Upon the stimulation of IL-17, IL-17R engages Act1 to mediate the recruitment of TRAF6. In all these cases, ubiquitination serves to recruit TAB2/TAB3/TAK1 and then NEMO/IKK kinase complexes, activating NF-κB signalling (right side of the figure). USPs including CYLD, USP7, USP15, USP16, USP19 and USP25 counteract the NF-κB signalling. CYLD removes Lys63-linked polyubiquitin chains from several substrates such as TNFR, TRADD, RIP1, TRAF2/5, cIAP1/2, MyD88 and TRAF6, negatively regulating NF-κB activation. USP15 deubiquitinates Lys48-linked polyubiquitination to maintain TAB2 stability and enhance NF-κB signalling induced by TNF and IL-1β. Similarly, upon TNF and IL-1β stimulation, USP19 removes Lys63- and Lys27-linked polyubiquitin chains from TAK1 and negatively regulates the activation of NF-kB. USP16 deubiquitinates Lys33-linked polyubiquitination from IKKβ and activates NF-kB. In IL-17 induced signalling, USP25 deubiquitinates Lys63-linked ubiquitination of TRAF6 mediated by Act1 and negatively regulates NF-κB signalling. Instead of regulating signals in the upstream of NF-κB, USP7 interacts with NF-κB subunits and deubiquitinates Lys48-linked ubiquitin chains, inhibiting p65 degradation and promoting NF-κB signalilng.