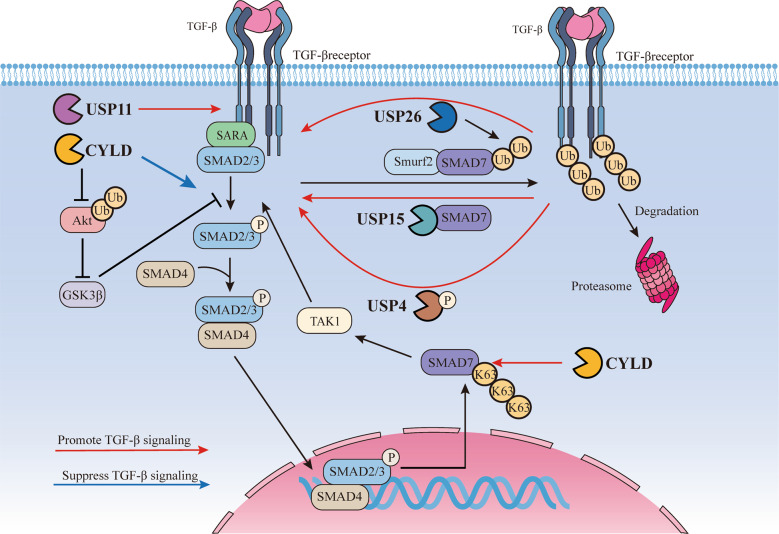
Fig. 3. USPs and TGF-β signalling.
TGF-β ligands bind to the TGF-β receptors and activate the SMADs proteins. USPs including USP4, USP11, USP15, USP26 and CYLD are associated with TGF-β signalling. Upon AKT-mediated phosphorylation, phosphorylated USP4 recruits to the activated TGF-β type I receptor directly and reverses receptor ubiquitination, leading to TGF-β signalling. USP11 deubiquitinates and stabilizes TGF-β type II receptor to promote TGF-β signalling. Reversed the polyubiquitination by SMAD specific E3 ubiquitin protein ligase 2 (Smurf2) and SMAD7, USP15 is recruited to the TGF-β type I receptor with scaffold protein SMAD7 as well and suppresses polyubiquitination and degradation of the receptor, positively regulating TGF-β signalling. USP26 is recognized as a negative regulator that deubiquitinates SMAD7 and stabilizes the interaction of SMAD7 and Smurf2, negatively regulating TGF-β signalling. CYLD decreases SMAD3 protein stability via an Akt-glycogen synthase kinase3β-hsc70-interacting protein-dependent manner, suppressing the activation of TGF-β signalling. CYLD also deubiquitinate Lys63-linked polyubiquitin chains of SMAD7 to regulate the activation of SMAD7-TAK1-TAB2/3 complex and transcription factor activator protein 1.