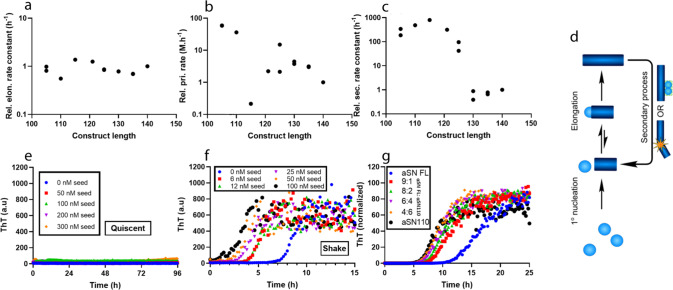
Fig. 2. Determination of individual rate constants of fibrillation for αSN truncated mutants.
a Relative elongation rate constant, k+, rel, obtained from seeded experiments shown in Fig. S6. There is little variation with length under the assumption that the length of the seed fibrils is similar for different truncation lengths. Details in Eq. 3. b The rate of primary nucleation, evaluated from the rate constant, kn, and reaction order, nc. Values calculated at 50 µM αSN to enable comparison between variants with varying reaction orders. c Rate constant of fragmentation, k-, versus construct length. There is a very abrupt decrease between αSN125 and αSN130. Each data point in a–c corresponds to a separate experiment with three repeats in three wells; αSN 105, αSN 125, aSN 130, and αSN 135 were randomly repeated. d Schematic reaction network with an explicit two-step mechanism for elongation. e, f Low-seed (0.03, 0.06, 0.1, 0.2%) fibrillation without (e) and with (f) shaking shows that shaking strongly promotes fibrillation. Data are for αSN121. (g) ThT fibrillation of different mixtures of αSN-FL and αSN110 (total protein concentration of 100 µM). Note that as little as 10% αSN110 significantly reduces the lag time. Shaking conditions in f, g are reduced to 1 min (instead of the 10 min used for the other shaking experiments) in 12-min measurement cycles.