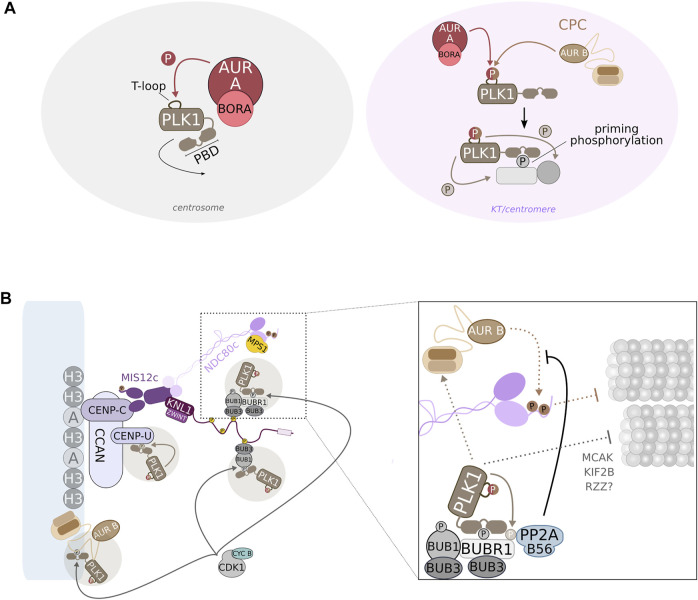
FIGURE 4.
POLO/PLK1 controls the turnover of KT-MT attachments. (A) At late G2, AURORA A:BORA phosphorylates PLK1 on its N-terminal T-loop to activate the kinase. At this stage, activation of PLK1 by AURORA A:BORA occurs at centrosomes, but subsequent maintenance of PLK1 activity at centromeres/KTs is likely maintained by AURORA B (although a residual fraction of AURORA A:BORA at KTs may also promoting PLK1 activation). Phosphorylation of the T-loop in PLK1 probably relieves an intramolecular interaction that mutually inhibits the N-terminal kinase domain and the C-terminal PBD. This can potentially contribute to fully activate PLK1 by exposing the kinase domain and allowing the PBD to interact with pre-phosphorylated (priming phosphorylation) substrates/binding partners. (B) PLK1 is recruited to multiple locations along the centromere/KT axis. CDK1:CYC B-mediated priming phosphorylation of PLK1 binding partners provides a positional cue for the localization of PLK1. However, PLK1 can promote its own recruitment by self-priming (ex, CENP-U/PBIP1). At KTs, PLK1 regulates the turnover of KT-MT interactions by promoting both the stabilization—PLK1-dependent phosphorylation of BUBR1 triggers the recruitment of PP2A:B56 phosphatase that, in turn, counteracts AURORA B activity—and destabilization of attachments—PLK1-dependent phosphorylation of KIF2B and MCAK likely promote MT depolymerization. PLK1 may also promote the destabilization of KT-MT attachments through an RZZ-dependent mechanism.