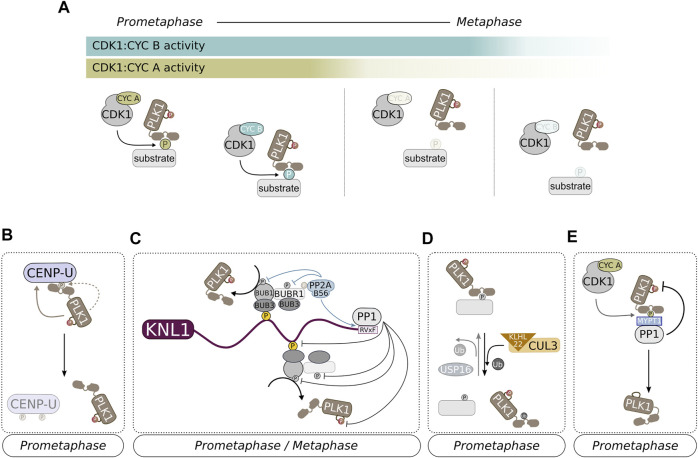
FIGURE 5.
Silencing of PLK1 activity coincides with chromosomes alignment during metaphase. (A) The degradation of CYCLIN A and CYCLIN B at the prometaphase-to-metaphase and metaphase-to-anaphase transitions, respectively, precludes the continuous priming of PLK1 substrates and contributes to silence the activity of the kinase at KTs. (B), (C), (D) and (E) Examples of other regulatory mechanisms to control PLK1 activity and function during prometaphase and metaphase. (B) PLK1 promotes its own release from KTs by targeting CENP-U/PBIP1 for degradation. (C) PP1 bound to KNL1 has been suggested to regulate PLK1 localization during metaphase by dephosphorylating key components required for PLK1 recruitment. A similar regulatory function has been proposed for PP2A:B56, which is recruited to KTs during prometaphase. (D) PLK1 localization is also regulated by non-proteolytic deubiquitination/ubiquitination of the PBD. (E) Other regulatory mechanisms control PLK1 activation status. For instance, CDK1:CYC A primes MYPT1, a PP1 regulatory subunit, and directs it to PLK1, thereby promoting PP1-mediated dephosphorylation of PLK1’s T-loop.