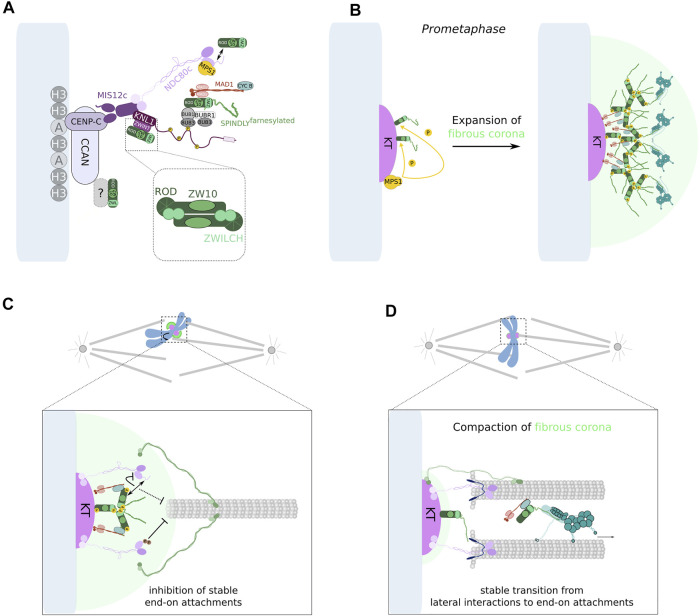
FIGURE 6.
RZZc:SPINDLY-dependent regulation of KT size dynamics and KT-MT attachment stability. (A) Localization of the RZZc to the outer KT requires different binding partners, including ZWINT and BUB1. Unlike RZZc:ZWINT, a direct interaction between the RZZc and BUB1 has not been demonstrated yet. Moreover, additional binding partners for the RZZc are likely to exist, but still remain unidentified (“?”). It has been proposed that NDC80 may constitute an RZZ binding partner, as ROD was found to interact with the N-terminal tail in NDC80 in a yeast two hybrid assay. Furthermore, the RZZc directly recruits SPINDLY to KTs. In human cells, this interaction requires SPINDLY to be farnesylated at its C-terminal CAAX motif (SPINDLYfarnesylated). (B) Expansion of the fibrous corona during prometaphase is driven by the RZZc:SPINDLYfarnesylated and is stimulated by MPS1-dependent phosphorylation of ROD. Importantly, SPINDLY also contributes to the subsequent compaction of the fibrous corona by recruiting DYNEIN:DYNACTIN. (C) The localization of different MAPs, including DYNEIN and CENP-E, to the fibrous corona facilitates initial MT capture. However, the presence of an expanded corona can inhibit the formation of stable load-bearing end-on attachments. The decreased stability of end-on attachments may result from the inhibitory action of the RZZc over NDC80/HEC1. (D) Compaction of the fibrous corona enables the conversion of lateral contacts between KTs and MTs into stable end-on attachments. DYNEIN:DYNACTIN has an critical function in efficiently stripping the RZZc away from KTs, thereby contributing to relieve its inhibitory activity towards KT-MT end-on attachments. DYNEIN:DYNACTIN-dependent disassembly of the fibrous corona also leads to the removal of SAC proteins and silencing of the mitotic checkpoint.