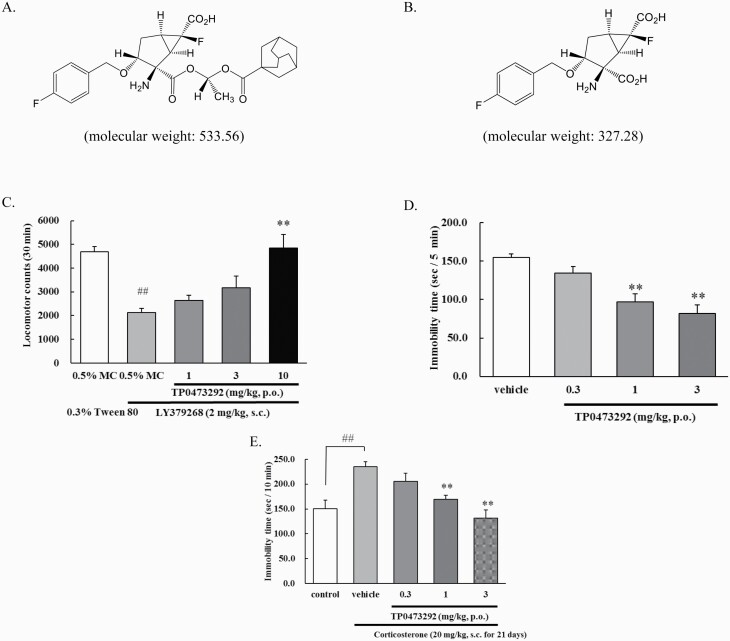
Figure 1.
Chemical structures of TP0473292 and TP0178894, and in vivo pharmacological profiles of TP0473292. (A) Chemical structure of TP0473292; (B) Chemical structure of TP0178894; (C) Effect of oral administration (p.o.) of TP0473292 on mGlu2/3 receptor agonist, LY379268-induced hypolocomotion in rats. Locomotor activity was measured for 30 min after subcutaneous (s.c.) administration of LY379268. Data represent mean ± SE (n = 8/group). ##P < .01 compared with 0.5% methyl cellulose 400 (MC) group (Student’s t-test), **P < .01 compared with LY379268-treated 0.5% MC group (Steel’s test); (D) Effect of oral administration (p.o.) of TP0473292 on immobility time in the forced swimming test of rats. Immobility time during the forced swimming test was measured for 5 min. Data represent mean ± SE (n = 9 – 10/group). **P < .01 compared with vehicle group (Dunnett’s test); (E) Effect of oral administration (p.o.) of TP0473292 on depressive-like behavior of corticosterone (CORT) treatment model of rats. Depressive-like behavior (increased immobility) was induced by treating rats with CORT (20 mg/kg) subcutaneously (s.c.) for 21 days, and the forced swimming test was conducted 2 days after the final CORT injection. Immobility time during the forced swimming test was measured for 10 minutes. Data represent mean ± SE (n = 8/group). ##P < .01 compared with control group (Student’s t test), **P < .01 compared with vehicle-treated group (Dunnett’s test).