Abstract
Background:
Patients with inflammatory bowel disease who achieve remission with anti-tumour necrosis factor (anti-TNF) drugs may have treatment withdrawn due to safety concerns and cost considerations, but there is a lack of prospective, controlled data investigating this strategy. The primary study aim is to compare the rates of clinical remission at 1 year in patients who discontinue anti-TNF treatment versus those who continue treatment.
Methods:
This is an ongoing, prospective, double-blind, multicentre, randomized, placebo-controlled study in patients with Crohn’s disease or ulcerative colitis who have achieved clinical remission for ⩾6 months with an anti-TNF treatment and an immunosuppressant. Patients are being randomized 1:1 to discontinue anti-TNF therapy or continue therapy. Randomization stratifies patients by the type of inflammatory bowel disease and drug (infliximab versus adalimumab) at study inclusion. The primary endpoint of the study is sustained clinical remission at 1 year. Other endpoints include endoscopic and radiological activity, patient-reported outcomes (quality of life, work productivity), safety and predictive factors for relapse. The required sample size is 194 patients. In addition to the main analysis (discontinuation versus continuation), subanalyses will include stratification by type of inflammatory bowel disease, phenotype and previous treatment. Biological samples will be obtained to identify factors predictive of relapse after treatment withdrawal.
Results:
Enrolment began in 2016, and the study is expected to end in 2020.
Conclusions:
This study will contribute prospective, controlled data on outcomes and predictors of relapse in patients with inflammatory bowel disease after withdrawal of anti-TNF agents following achievement of clinical remission.
Clinical trial reference number:
EudraCT 2015-001410-10
Keywords: anti-tumour necrosis factor, inflammatory bowel disease, withdrawal
Introduction
Anti-tumour necrosis factor (anti-TNF) agents such as infliximab and adalimumab are recommended therapies for the medical management of moderate to severe inflammatory bowel disease (IBD), that is, Crohn’s disease and ulcerative colitis, particularly in patients who are steroid-refractory or steroid-dependent.1,2 In addition to being effective for the induction of remission, anti-TNF agents are also used for maintenance therapy in patients with IBD.1,2 Although these agents have been shown to be effective at maintaining remission, promoting mucosal healing and improving disease course and long-term outcomes,1–4 long-term therapy might be associated with safety concerns, such as possible increased risks of opportunistic infection and malignancy, and a substantial cost. 4 Thus, it has been suggested that, after a period of stable remission, anti-TNF maintenance therapy could be discontinued in some patients.
Withdrawal of anti-TNF therapy may be associated with a risk of relapse. A meta-analysis of 27 studies found that the risk of relapse 1 year following discontinuation of anti-TNF treatment was 40% for patients with Crohn’s disease and 28% for those with ulcerative colitis. 5 In a retrospective study of 1055 patients who had anti-TNF therapy withdrawn after achieving clinical remission, the rate of relapse was 19% per patient-year in patients with Crohn’s disease, and 17% per patient-year in patients with ulcerative colitis. 6 Although some factors, such as mucosal healing, 7 appear to be associated with lower risk of relapse, investigations into the rate of relapse after anti-TNF discontinuation and the associated predictive factors is confounded by differences in study design, the retrospective design of most of the studies and insufficient data to allow assessment of all potential contributing factors.5–7 In addition, many of the studies investigating outcomes following anti-TNF withdrawal enroll only a small number of patients.8–12 Thus, prospective controlled data from a large, appropriately powered population are clearly needed.
It is noteworthy that there are no randomized clinical trials comparing anti-TNF discontinuation with continuation in patients with IBD. Patients who continue treatment can also experience relapse, due to loss of efficacy of long-term anti-TNF treatment, thus it is crucial that any study of anti-TNF withdrawal includes a control group, in order to compare the rate of relapse in those continuing and discontinuing therapy. 13
The aim of the present study is, therefore, to evaluate the risk of relapse in patients with IBD who discontinue anti-TNF therapy after achieving remission in comparison with those who continue receiving treatment, in a randomized controlled trial. In addition, we aim at identifying predictors of relapse, in order to select patients who can safely discontinue biologic therapy with low risk of relapse. We anticipate that the results provided by this trial will have a significant impact on how patients with IBD are managed in clinical practice.
Methods
Study design
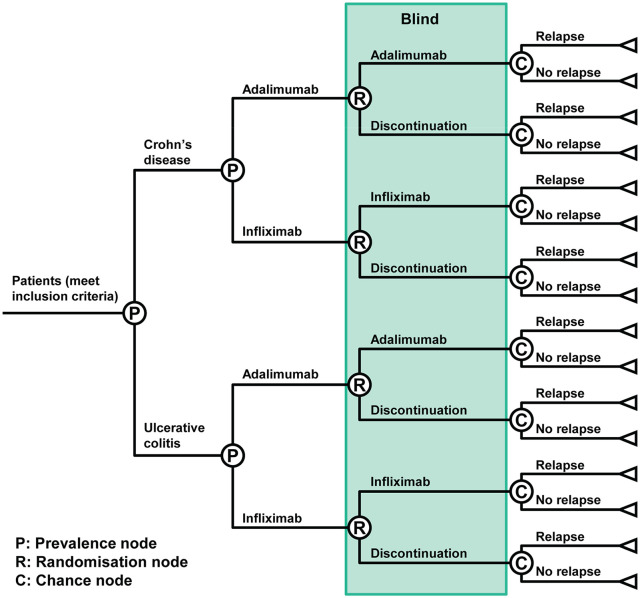
This study was designed to prospectively investigate the effect of anti-TNF discontinuation on the risk of relapse in patients with IBD. A quadruple-blind, randomized study design was chosen, in which the patient, physician, data manager and statistician were all blinded to the patient’s treatment allocation. The study is being conducted at 47 IBD hospital units across Spain, with enrolment starting in 2016 and continuing until 2019. The study is expected to end in 2020, when the last enrolled patient will complete the final follow-up visit. There are two strategies that are being compared: discontinuation of anti-TNF treatment versus continuation of anti-TNF after achieving remission, as shown in Figure 1. Patients are being randomized to one of two groups (anti-TNF continuation or placebo) using a decision-tree type format and an interactive web response system. Randomization is stratified by the type of IBD (Crohn’s disease or ulcerative colitis), and the anti-TNF agent (infliximab or adalimumab) at the time of study inclusion (Figure 1).
Figure 1.
Randomization scheme.
The primary objective of this study is to compare the rates of clinical remission at 1 year in patients who discontinue treatment with an anti-TNF agent versus those who continue. Secondary endpoints being compared between the two groups include: the duration of relapse-free time; mucosal healing; quality of life (QoL) and work productivity; safety; and the identification of predictive factors for relapse.
As described above, the quadruple-blind study design renders the patient, physician, data manager and statistician blind to the study treatment. Only the pharmacy staff and the nurse responsible for study drug administration know the treatment assigned to any given patient.
Ethics approval for this study has been granted by a Central Clinical Research Ethics Committee at the Hospital Universitario de La Princesa. The trial is being conducted according to the ethical principles set out in the Declaration of Helsinki and relevant Spanish law regarding clinical trials. It is registered in both European and United States clinical trial registers (EudraCT number 2015-001410-10, and ClinicalTrials.gov identifier NCT02994836). Written, informed consent is being obtained from all participating patients.
Treatment arms
Patients in group 1 continue their current anti-TNF treatment (infliximab or adalimumab) while patients in group 2 are given a placebo matched to the drug they were previously receiving. That is, patients who were on infliximab receive an intravenous placebo, while patients who were receiving adalimumab receive a placebo administered subcutaneously. The placebo for infliximab is provided in the same pharmaceutical form and route of administration as the active drug, while the syringes for adalimumab and its placebo are masked so they cannot be seen by the patient when the nurse is administering them. Infliximab (5 mg/kg) or infliximab placebo is administered every 8 weeks, while adalimumab (40 mg) or its placebo is administered every 2 weeks. Patients in both groups will continue to receive their usual immunosuppressant therapy (thiopurine or methotrexate).
Patients
Patients are eligible for enrolment in this study if they are aged over 18 years with IBD (either Crohn’s disease or ulcerative colitis) diagnosed according to the European Crohn’s and Colitis Organisation (ECCO) criteria and receiving treatment with an anti-TNF biologic (infliximab or adalimumab) and an immunosuppressant. Patients with Crohn’s disease are eligible only if their anti-TNF treatment was prescribed for luminal involvement (not perianal). At the time of inclusion, patients must be in steroid-free clinical remission (see definition in the section entitled ‘Endpoints’) for at least 6 months at a nonintensified dose of anti-TNF and also be receiving concomitant immunosuppressants (thiopurine or methotrexate) at stable doses for at least 3 months prior to inclusion in the study. Intensified doses of anti-TNF therapy are defined as ⩾10 mg/kg/8 weeks or 5 mg/kg/⩽4 weeks for infliximab and 40 mg/week for adalimumab. Patients must have had a colonoscopy, or magnetic resonance enterography (MRE) for patients with ileal or ileocolic Crohn’s disease, within 3 months of study inclusion, and this baseline assessment must not show significant lesions (see definition in the section entitled ‘Assessments’).
Exclusion criteria include: age <18 years; receiving anti-TNF therapy for a non-IBD indication; Crohn’s disease treated with anti-TNF agents for perianal involvement (or both perianal and luminal); no concomitant treatment with immunosuppressants (thiopurine or methotrexate) at the time of enrolment and within the prior 3 months; history of bowel resection surgery; presence of significant endoscopic or radiological lesions; advanced chronic disease or any other condition that results in an inability to attend the clinic for monitoring or follow up; women who are pregnant or breastfeeding, or who intend to become pregnant during the study period; and refusal to consent to study participation.
Endpoints
The primary endpoint of the study is ‘sustained clinical remission at 1 year’ after randomization. Any need for intensification of anti-TNF, modification of concomitant treatment, or therapy addition to maintain clinical remission is considered as failure to maintain remission. Clinical remission for patients with Crohn’s disease is defined as a Crohn’s Disease Activity Index (CDAI) score of <150 points, 14 while for patients with ulcerative colitis remission is defined as a Partial Mayo Score (PMS) of ⩽2, with all scores from the partial score being ⩽1, and a rectal bleeding subscore of 0. 14 Clinical relapse is defined as a CDAI > 150 points or a PMS > 2 (as applicable) in two consecutive visits, separated by at least 1 week. If a patient has a clinical relapse after randomization, the responsible physician will take any measure deemed necessary to manage the relapse.
Other endpoints include endoscopic activity, radiological activity, patient-reported outcomes, QoL, work productivity and safety.
Assessments
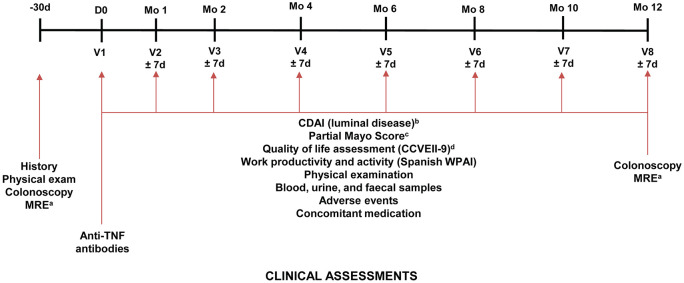
The timeline for study visits conducted at each blinded study visit is presented in Figure 2.
Figure 2.
Schedule of study visits throughout the study. CCVEII-9, ‘Cuestionario de Calidad de Vida Específico para la Enfermedad Inflamatoria Intestinal-9’ (Questionnaire on Quality of Life for Inflammatory Bowel Disease-9).
CDAI, Crohn’s Disease Activity Index; d, day; IV, intravenous; mo, month; MRE, magnetic resonance enterography; SC, subcutaneous; TNF, tumour necrosis factor; V, visit; WPAI, Work Productivity and Activity Index.
aPatients with ileal or ileocolic Crohn’s disease.
bPatients with Crohn’s disease.
cPatients with ulcerative colitis.
dA shortened version of the CCVEII-9 questionnaire is being used.
Clinical activity assessment
Clinical activity was assessed at study visits (Figure 2) using the CDAI in patients with Crohn’s disease and the PMS in patients with ulcerative colitis.
Radiological assessment
In patients with ileal or ileocolic Crohn’s disease participating in the study, disease activity is assessed by MRE at baseline and at the end of the study period. If the patient has had a previous MRE within 3 months of the baseline visit that contains all the information required for the study, that MRE can be used for study inclusion. Absence of activity in the small intestine or colon is defined as the absence of contrast enhancement, oedema, or ulcers. A thickening of the wall without enhancement is considered as remission. Significant radiological lesions by MRE are defined as the presence of oedema in T2 or ulcers in two or more intestinal segments (rectum, descending colon, transverse colon, ascending colon, ileum).
Endoscopic assessment
All patients participating in the study have to undergo a baseline colonoscopy, but if the patient had a colonoscopy within 3 months of the baseline visit and this contains all of the information required for the study, it will be acceptable for patient inclusion. A second colonoscopy will be performed in all patients at the end of the assessment period.
At each endoscopic assessment in patients with Crohn’s disease, disease activity is being assessed using the Simplified Endoscopic Activity Score for Crohn’s Disease (SES-CD), 15 with significant endoscopic lesions defined as the presence of any of the following: an SES-CD score of ⩾5, any deep ulcer, or superficial ulcers covering >10% of the surface of at least one intestinal segment. For patients with ulcerative colitis, endoscopic activity is being assessed using the Mayo Endoscopic Subscore; 16 a Mayo Endoscopic Subscore of 3 is considered to indicate the presence of significant endoscopic lesions in these patients. The assessment of endoscopic activity before study entry and at the end of follow up is being performed by the investigator, as well as centrally by an investigator who assesses anonymised endoscopic images of the ileum (in patients with Crohn’s disease) and of each colonic segment scanned.
Patient-reported outcomes
Assessment of QoL is being undertaken using the shortened CCVEII-9 QoL questionnaire 17 and assessment of work productivity and activity is being assessed using a Spanish version of the Work Productivity and Activity Impairment (WPAI) questionnaire.18,19
Laboratory assessments
Blood samples are being taken for haematology and biochemistry profiles, as well as cytokine profiling, drug (trough) levels and antidrug antibodies. In addition, faecal and urinary samples are being obtained at baseline and at every visit during follow up; biomarkers associated with IBD recurrence will be investigated in those samples.
Safety endpoints
Information on toxicity/adverse events (AEs) is being collected at all study visits via spontaneous patient reporting and patient interviews at each visit. AEs, adverse drug reactions (ADRs) and reports of pregnancy are being collected from the time the patient provides their informed consent until up to 30 days after the last dose of study drug or the last visit. All AEs and ADRs are assigned an intensity (mild, moderate or severe), and AEs are being classified by the principal investigator according to their causal relationship to the study drug (certain, probable, possible, conditional or unlikely, or unrelated). All AEs are being followed until their resolution or for at least 30 days after study drug discontinuation, until toxicity is ⩽1 or it is deemed irreversible. Similarly, any pregnancies occurring during the study will be followed and their outcomes recorded, in order to rule out any abnormalities or congenital malformations.
Data collection
Study data are being collected and managed using an electronic data capture tool [Research Electronic Data Capture (REDCap)], 20 which is hosted at Asociación Española de Gastroenterología (AEG; www.aegastro.es), a nonprofit medical society. AEG provides this service free of charge, with the aim of promoting investigator-driven research. REDCap is a secure, web-based application designed to support data capture for research studies that provides the following: an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources. 20
Statistical analysis
Sample size calculation
We estimated that 194 patients (97 patients in each group) would be required to compare the effect of anti-TNF withdrawal versus continuation. This is based on the assumption that 10% of patients continuing anti-TNF treatment for longer than 1 year will experience a loss of efficacy at 1 year, 21 while the incidence of relapse after anti-TNF discontinuation is expected to be 25% at 1 year. 6 Therefore, 194 patients would be required to demonstrate statistically significant differences between the two strategies with 80% power at a 5% significance level.
Description of intention-to-treat and per-protocol populations
Patients who meet the inclusion criteria and have been randomized are included in the intention-to-treat (ITT) analysis irrespective of whether they strictly adhere to the protocol. The per-protocol (PP) analysis will include only those patients who have completed treatment and follow up according to the protocol. Patients who provide signed informed consent but do not receive the first dose of study medication for any reason are being replaced to maintain the required sample size. Patients withdrawing due to pregnancy will be considered as failure in the ITT analysis, and as not assessable in the PP analysis.
Data presentation and analyses
Qualitative variables will be presented as percentages with 95% confidence intervals, while quantitative variables will be presented as means and standard deviations (normal distribution) or medians and interquartile ranges (non-normal distribution). Categorical variables will be compared using the Chi-square test and quantitative variables by the appropriate test for their distribution (Student’s t test or Wilcoxon test). Mean and median time to relapse in the anti-TNF discontinuation versus continuation groups will be compared.
The Kaplan–Meier method will be used to evaluate recurrence-free time, and the log-rank test will be used to assess differences between the anti-TNF discontinuation or continuation curves. The variables associated with IBD recurrences following treatment discontinuation will be analysed using a Cox regression model, with treatment strategy (anti-TNF discontinuation versus continuation) as an independent variable, and including other factors possibly related to an increased recurrence risk, such as age, sex, smoking, type of IBD, infliximab trough levels and indication for anti-TNF treatment.
Discussion
The worldwide burden of IBD is significant, and is particularly high in Europe and North America. 22 According to a review of published epidemiological studies, the prevalence of Crohn’s disease in Europe ranges from 1.51 cases per 100,000 people in Romania to 322.0 cases per 100,000 in Germany. 22 The prevalence of ulcerative colitis is slightly higher, ranging from 2.42 per 100,000 (again in Romania) to 505.0 per 100,000 in south-eastern Norway. 22 Overall, an estimated 0.3% of the European population is affected by IBD, equating to around 2.5–3 million people. 23
The onset of IBD is usually during adolescence or early adulthood, so there is considerable cumulative healthcare resource use associated with this disease over the lifetime of a patient. 24 Although for most patients, IBD follows a relapsing–remitting course, between 20% and 25% of European patients will have continuous chronic symptoms. 23 In addition, as many as 20–40% of patients with Crohn’s disease and 15–20% with ulcerative colitis have extraintestinal manifestations, including arthropathies, ocular and skin conditions. 23 According to estimates by the ECCO, IBD was associated with direct healthcare costs of between €4.6 billion and €5.6 billion per year in 2013. 23 In addition to the direct healthcare costs, IBD is associated with lost productivity when patients are unable to work or pursue their education because of their symptoms.23,24 As well as impacting the patient economically, the inability to work has a significant negative effect on their QoL. 25
The introduction of biologic therapy has not only changed treatment patterns for IBD, but also the type of costs incurred. Before the widespread use of biologic therapy, hospitalization and surgery accounted for the greatest portion of treatment costs, but today medication makes up the greatest proportion of the total cost. 24 According to 2016 estimates, the cost of biologic therapy varies widely across Europe (the lowest annual cost per patient is approximately €6000 for an infliximab biosimilar). 26 In many European countries, 100% of these costs are borne by the public healthcare system; in others, most of the cost is borne by the public healthcare system, with patients contributing a small co-pay of 10–25%. 26 Therefore, it is important from a health economic perspective to determine whether these agents can be safely discontinued in patients who have achieved a stable remission. Furthermore, since long-term use of these agents can be associated with the risk of malignancy and opportunistic infections, 4 it is also important to determine whether discontinuation of anti-TNF agents can reduce such risks.
The current study was designed to provide robust evidence about the effect of anti-TNF discontinuation on the natural history of IBD. While studies and analyses exist demonstrating that a proportion of patients relapse after withdrawal of anti-TNF treatment following clinical remission,5,6,8–12 identification of predictors of response and extrapolation of these data to the broader IBD population is hampered by different study designs, small populations, and variable results. In addition, to the best of our knowledge there is currently no published randomized, controlled trial comparing patients continuing anti-TNF treatment with those discontinuing treatment. The data obtained in this study will contribute to resolving some of the uncertainties remaining in this controversial aspect of IBD management.
In conclusion, no randomized clinical trial has yet been undertaken to compare the strategy of discontinuing anti-TNF treatment with maintenance of these drugs in patients with IBD. Therefore, it has not been possible to adequately compare the natural history of the condition in the absence versus presence of anti-TNF maintenance therapy, particularly since the latter group of patients may also experience disease relapses due to the loss of effectiveness of long-term anti-TNF treatment. The current study was designed to address the existing knowledge gap about the clinical impact of withdrawing anti-TNF therapy in patients with IBD who have achieved a sustained remission. By incorporating robust research techniques, including randomization, the use of a control group, and blinding, this study will provide important information on the management of patients with IBD and how to manage the growing burden of this illness within the constraints of a public healthcare system.
Acknowledgments
The authors wish to thank A.G. McNicholl and J. Calvo for programming the e-CRF in AEG-REDCap (from the Spanish Association of Gastroenterology) as well as the Spanish Clinical Research Network, from the Instituto de Salud Carlos III, for data monitoring.
We would like to thank the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) for the critical review of the protocol by the scientific evaluation committee, and for the support of the scientific secretariat.
We would like to thank Eva Rodríguez, Project Manager of the study and scientific secretary of GETECCU. We would also like to thank Sheridan Henness, who provided assistance with manuscript development on behalf of Springer Healthcare Communications. This editorial assistance was funded by Fundación de Investigación Biomédica, Hospital Universitario de La Princesa.
J.P. Gisbert and M. Chaparro designed the study and are leading its development. All other authors contributed to study design and critically revised the protocol, and were involved in patient enrolment and follow up.
J.P. Gisbert and M. Chaparro are the guarantors of the article.
List of collaborators: F. Abad, M. Aguas Peris, E. Agüero Tejado, C. Alba, M. Albert, H. Alemán, A. Algaba, I. Alonso Abreu, M.P. Amador, M. Amat, T. Angueira, C. Arajol, L. Arias-González, A. Arrondo Velasco, M. Baldán, B. Bardán García, A. Bargalló García, M. Barreiro de Acosta, J. Barrio Andrés, G. Bastida Paz, I. Bastón Rey, L. Batista, M. Bellver Martínez, B. Beltrán Niclós, J.M. Benítez, Y. Ber Nieto, F. Bermejo, D. Bernardo, I. Blázquez Gómez, A. Bouhmidi Assakali, D. .Busquets Casals, J.L. Cabriada Nuño, X. Calvet Calvo, M.V. Calvo Hernández, M. Calvo, B. Camps, A.Y. Carbajo, G. Cardona Peitx, T. Caro-Patón, M. Carrillo Palau, S. Carrión Bolorino, M.J. Casanova, J.A. Casellas Valdé, A. Castaño García, B. Castro Senosiain, D. Ceballos, E. Cerrillo, S. Chacón Martínez, F. Consuelo Cañete Pizarro, M.L. de Castro Parga, M. de Miguel, R. de Francisco García, M.D. de la Cruz Ramírez, J. del Hoyo Francisco, P. Delgado Guillena, T. Desongles Corrales, A. Echarri Piudo, E. Espino Paisan, M. Espona Quer, M. Esteve, A. Fernández Pordomingo, J.L. Fernández Forcelledo, S. Fernández-Tomé, R. Ferreiro Iglesias, I. Ferrer Bradley, A. Ferrer, A. Figueroa, M. Gallach Montero, P. García Iglesias, C. García García-Lezcún, L. García Ramírez, M.J. García García, O. García-Bosh, A. Garre, T. Giménez Poderós, L. Gómez Irwin, B. Gómez Pastrana, E. Gómez Delgado, Y. González Lama, Á. Gracia García, B. Gracia García, J. Guardiola, I. Guerra, E. Guerra, V. Guillot, S. Gustmancher Saiz, A. Gutiérrez Casbas, V. Hernández Ramírez, M.M. Hernando Verdugo, B. Hernández Muniesa, R. Hernanz Chaves, J.M. Herrera Justiniano, J Hinojosa del Val, S Ibáñez Feijoo, M Iborra Colomino, E Iglesias Flores, E. Izquierdo García, M J Sampedro González, A J. Lucendo, N Jiménez García, E. Leo Carnerero, I. Loizaga Díaz, A López de Torre Querejazu, P López Sánchez, J Luis Parras, M Maia Boscá, M Mañosa, S Marín Pedrosa, A Marín, Á Marinero, I Marín-Jiménez, L Márquez Mosquera, JL Márquez Galán, E Martín Arranz, MD Martín Arranz, J Martínez Cadilla, JM Martínez Sesmero, B Martínez Sánchez, V Matallana, MI Mateos Hernández, AG McNicholl, R Mejuto Fernández, L Melcarne, L Menchén, J Méndez-Castrillón Rodríguez, O Merino Ochoa, M Mínguez, G Molas Ferrer, M Montoro Huguet, A Montserrat Torres, F Mora, I Moraleja Yudego, VJ Morales Alvarado, L Morales Martínez, A Morell, C Motos García, F Muñoz Alonso, MC Muñoz Villafranca, JE Muñoz, A Mur, Ó Nantes, P Navarro, M Navarro-Llavat, P Nos Mateu, A Núñez Alonso, A Núñez Ortiz, D Olivares, V Ollero Pena, J Orobitg, L Ortega, J Ortiz de Zárate, H Pallarés Manrique, A Paradela Carreiro, L Peral Ballester, S Pereira Bueno, I Pérez Martínez, JR Pineda Mariño, C Piñero Pérez, A Planas Giner, MR Plaza Santos, Á Ponferrada Díaz, J Poza Cardón, V Prieto Vicente, L Puchades, L Ramos López, S Redondo, S Riestra Menéndez, M Rivero Tirado, I Rodríguez Lago, C Rodríguez Gutiérrez, E Rodríguez, S Romero Izquierdo, S Rubio Iturria, MB Ruiz Antorán, A Ruiz, LF Salazar, A Sánchez Ulayar, E Sánchez Gómez, C Sánchez, C Sangrador, K Serra, K Spicakova, C Suárez Ferrer, A Talavera Fabuel, C Taxonera, M Tordera, E Torrella Cortés, J Tosca, C Trigo Salado, F Uriarte Estefanía, M Van Domselaar, JM Vázquez Morón, P Ventura López, M Vera, M Vicuña Arregui, A Villoria Ferrer, T Virgós Aller, D Yáñez Feria.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the Instituto de Salud Carlos III (PI15/00560). Project financed with FEDER funds.
Conflict of interest statement: J.P. Gisbert has served as a speaker, a consultant and advisory member for or has received research funding from AbbVie, Advia, Allergan, Almirall, AstraZeneca, Biogen, Casen Fleet, Casen Recordati, Celgene, Chiesi, Diasorin, Dr. Falk Pharma GmbH, Faes Farma, Ferring Pharmaceuticals, Gebro Pharma, Janssen, Hospira, Kern Pharma, Mayoly, MSD, Nycomed, Otsuka Pharmaceutical, Pfizer, Roche, Sandoz, Shire Pharmaceuticals, Takeda, Tillotts Pharma AG and Vifor Pharma.
M. Chaparro has served as a speaker, or has received research or education funding from AbbVie, Dr. Falk Pharma GmbH, Ferring Pharmaceuticals, Janssen, Hospira, MSD, Pfizer, Shire Pharmaceuticals, Takeda and Tillotts Pharma AG.
M. Barreiro-de Acosta has served as a speaker, a consultant and advisory member for or has received research funding from AbbVie, AMGEN, Chiesi, Dr. Falk Pharma GmbH, Faes Farma, Ferring Pharmaceuticals, Gebro Pharma, Janssen, Hospira, Kern Pharma, MSD, Otsuka Pharmaceuticals, Pfizer, Sandoz, Shire Pharmaceuticals, Takeda and Tillotts Pharma AG.
E. Domènech has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Celgene, Ferring Pharmaceuticals, Gebro Pharma GmbH, Grifols, Janssen, Kern Pharma, MSD, Otsuka Pharmaceuticals, Pfizer, Shire Pharmaceuticals, Takeda, Thermofisher and Tillots Pharma AG.
M. Esteve reports personal fees from Janssen, Menarini, Pfizer, Takeda and Tillots Pharma AG, and grants from AbbVie and MSD, outside the submitted work.
P. Nos has participated in educational activities, research projects and scientific meetings sponsored by AbbVie, Faes Farma, Ferring Pharmaceuticals, Janssen, MSD, Otsuka, Takeda and Tillots Pharma AG.
J. Panés has received consulting fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals, Genentech, GlaxoSmithKline, Janssen, MSD, Nestle, Oppilan, Progenity, Pfizer, Robarts, Roche, Second Genome, Takeda, Theravance, TiGenix and Topivert; speaker fees from AbbVie, Biogen, Ferring Pharmaceuticals, Janssen, MSD, Shire Pharmaceuticals, Takeda and Tillotts Pharma AG; and research funding from AbbVie and MSD.
ORCID iD: María Chaparro  https://orcid.org/0000-0002-9275-4242
https://orcid.org/0000-0002-9275-4242
Contributor Information
María Chaparro, Gastroenterology Department, Hospital Universitario de La Princesa, IIS-IP, Universidad Autónoma de Madrid, CIBEREHD, Madrid, Spain.
María G. Donday, Gastroenterology Department, Hospital Universitario de La Princesa, IIS-IP, Universidad Autónoma de Madrid, CIBEREHD, Madrid, Spain
Manuel Barreiro-de Acosta, Gastroenterology Department, Hospital Clínico Universitario de Santiago de Compostela, Spain.
Eugeni Domènech, Gastroenterology Department, Hospital Germans Trias i Pujol, CIBEREHD, Badalona, Spain.
María Esteve, Gastroenterology Department, Hospital Universitari Mútua Terrasa, CIBEREHD, Barcelona, Spain.
Valle García-Sánchez, Gastroenterology Department, Hospital Universitario Reina Sofía, Córdoba, Spain.
Pilar Nos, Gastroenterology Department, Hospital Universitario y Politécnico La Fe, CIBEREHD, Valencia, Spain.
Julián Panés, Gastroenterology Department, IBD Unit, Hospital Clínic, IDIBAPS, CIBEREHD, Barcelona, Spain.
Concepción Martínez, Gastroenterology Department, Servicio de Farmacia, Hospital Universitario de La Princesa, Madrid. Spain.
Javier P. Gisbert, Gastroenterology Department, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS-IP), Universidad Autónoma de Madrid, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Diego de Leon 62, Madrid, 28006, Spain.
References
- 1. Gomollón F, Dignass A, Annese V, et al. Third European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 2. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 3. Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology 2005; 128: 862–869. [DOI] [PubMed] [Google Scholar]
- 4. Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2014; 40: 338–353. [DOI] [PubMed] [Google Scholar]
- 5. Gisbert JP, Marin AC, Chaparro M. The risk of relapse after anti-TNF discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2016; 111: 632–647. [DOI] [PubMed] [Google Scholar]
- 6. Casanova MJ, Chaparro M, Garcia-Sanchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol 2017; 112: 120–131. [DOI] [PubMed] [Google Scholar]
- 7. Gisbert JP, Marin AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther 2015; 42: 391–405. [DOI] [PubMed] [Google Scholar]
- 8. Farkas K, Lakatos PL, Nagy F, et al. Predictors of relapse in patients with ulcerative colitis in remission after one-year of infliximab therapy. Scand J Gastroenterol 2013; 48: 1394–1398. [DOI] [PubMed] [Google Scholar]
- 9. Helwig U, Lutter F, Koppka N, et al. Proposal for an anti-TNF-exit strategy based on trough serum level. Biologicals 2017; 47: 81–85. [DOI] [PubMed] [Google Scholar]
- 10. Hlavaty T, Krajcovicova A, Letkovsky J, et al. Relapse rates of inflammatory bowel disease patients in deep and clinical remission after discontinuing anti-tumor necrosis factor alpha therapy. Bratisl Lek Listy 2016; 117: 205–211. [DOI] [PubMed] [Google Scholar]
- 11. Legue C, Brochard C, Bessi G, et al. Outcomes of perianal fistulising Crohn’s disease following anti-TNFalpha treatment discontinuation. Inflamm Bowel Dis 2018; 24: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 12. Molander P, Farkkila M, Kemppainen H, et al. Long-term outcome of inflammatory bowel disease patients with deep remission after discontinuation of TNFalpha-blocking agents. Scand J Gastroenterol 2017; 52: 284–290. [DOI] [PubMed] [Google Scholar]
- 13. Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009; 104: 760–767. [DOI] [PubMed] [Google Scholar]
- 14. D’haens G, Feagan B, Colombel JF, et al. Challenges to the design, execution, and analysis of randomized controlled trials for inflammatory bowel disease. Gastroenterology 2012; 143: 1461–1469. [DOI] [PubMed] [Google Scholar]
- 15. Daperno M, D’haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 16. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 17. Alcala MJ, Casellas F, Fontanet G, et al. Shortened questionnaire on quality of life for inflammatory bowel disease. Inflamm Bowel Dis 2004; 10: 383–391. [DOI] [PubMed] [Google Scholar]
- 18. Vergara M, Montserrat A, Casellas F, et al. Validation of the Spanish Work Productivity and activity impairment questionnaire: Crohn’s disease version. Eur J Gastroenterol Hepatol 2009; 21: 809–815. [DOI] [PubMed] [Google Scholar]
- 19. Vergara M, Montserrat A, Casellas F, et al. A new validation of the Spanish work productivity and activity impairment questionnaire-Crohn’s disease version. Value Health 2011; 14: 859–861. [DOI] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaparro M, Panes J, Garcia V, et al. Long-term durability of infliximab treatment in Crohn’s disease and efficacy of dose “escalation” in patients losing response. J Clin Gastroenterol 2011; 45: 113–118. [DOI] [PubMed] [Google Scholar]
- 22. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 23. Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013; 7: 322–337. [DOI] [PubMed] [Google Scholar]
- 24. Cohen RD. The pharmacoeconomics of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol 2010; 7: 103–109. [DOI] [PubMed] [Google Scholar]
- 25. Hoivik ML, Bernklev T, Solberg IC, et al. Patients with Crohn’s disease experience reduced general health and vitality in the chronic stage: ten-year results from the IBSEN study. J Crohns Colitis 2012; 6: 441–453. [DOI] [PubMed] [Google Scholar]
- 26. Pentek M, Lakatos PL, Oorsprong T, et al. Access to biologicals in Crohn’s disease in ten European countries. World J Gastroenterol 2017; 23: 6294–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]