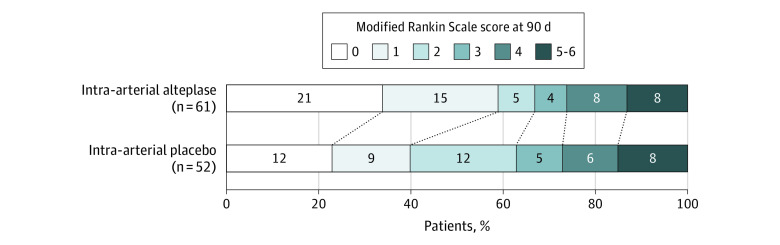
Figure 2. Distribution of Functional Scores at 90 Days in the CHOICE Trial of Intra-arterial Alteplase.
Scores on the modified Rankin Scale for patients in the intra-arterial alteplase group (n = 61) and the placebo group (n = 52) who were evaluated by local investigators via face-to-face interview. Scores range from 0 to 6, with 0 indicating no symptoms; 1, no clinically significant disability; 2, slight disability (able to handle own affairs without assistance but unable to carry out all previous activities); 3, moderate disability (requiring some help, but able to walk unassisted); 4, moderately severe disability (unable to attend body needs and unable to walk); 5, severe disability (requiring constant nursing care and attention); and 6, death. Scores of 5 and 6 were combined for the analysis. Treatment with intra-arterial alteplase was associated with a favorable outcome (a score of 0 or 1 on the modified Rankin Scale) at 90 days, with an adjusted risk difference of 18.4% (95% CI, 0.3%-36.4%; P = .047). The difference between the intra-arterial alteplase group and the placebo group in the overall distribution of scores was not statistically significant (shift analysis, adjusted common odds ratio for worsening of 1 point on the modified Rankin Scale, 1.54; 95% CI, 0.79-2.94).