FIGURE 10.
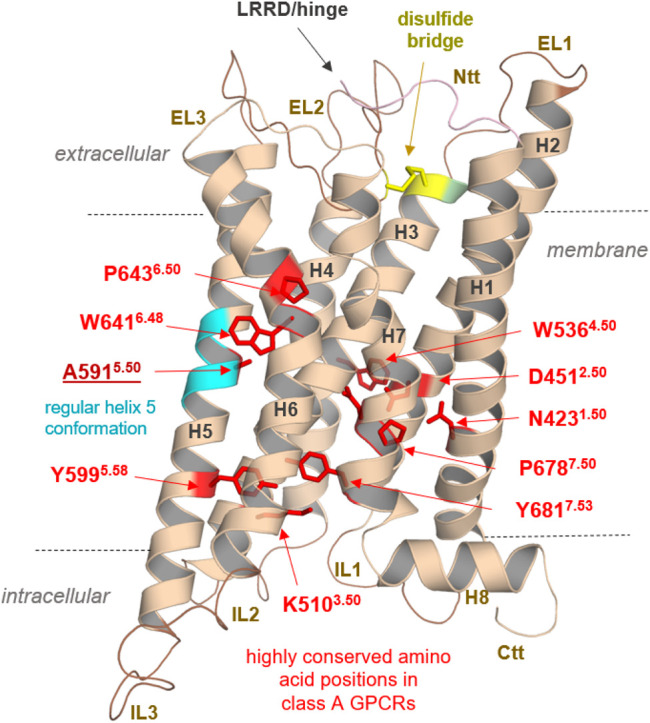
Specificities of RXFP1 and common class A GPCR properties. Typical highly conserved class A GPCR amino acids are highlighted as red sticks in the TMD model of the RXFP1. They are responsible for specific receptor functions as structural fold, expression, and signal transduction. In addition, they determine structural features in the helices (as the prolines, e.g., P6436.50), or they participate as intramolecular switches (as W6416.48) regulating the transition between active and inactive state conformations (D4512.50). Of note, the TMH5 conformation is likely regular compared to most other class A GPCRs with a proline-induced kink (cyan helix region) because RXFP1 has an alanine at this position. Superscripted numbers according to the unifying Ballesteros & Weinstein numbering for class A GPCRs (Ballesteros and Weinstein, 1995). An additional structural factor of importance shared between the LGRs is homo- or hetero-oligomerization, a widely recognized property of many GPCRs (e.g., (Smith and Milligan, 2010; Tena-Campos et al., 2014)), including receptors of relevance in endocrinology (Kleinau et al., 2016). Cooperative effects between receptor monomers arranged as dimers or oligomers (homomers) are long known to be obligatory and impact LGR groups A and C ligand binding properties (Chazenbalk et al., 1996; Urizar et al., 2005; Svendsen et al., 2009; Zoenen et al., 2012). Furthermore, interactions with non-LGR receptors (heteromerization), such as angiotensin receptors (AT1R and AT2R), have been identified in vivo, specifically for RXFP1 (Chow et al., 2014; Chow et al., 2019). This fact drastically widens the spectrum of the potential functional and physiological importance of this receptor. Finally, the RXFP1 is a highly interesting and pharmacologically relevant class A GPCR with unique structural and functional features, which needs further advanced experimental elucidation.
