Figure 11.
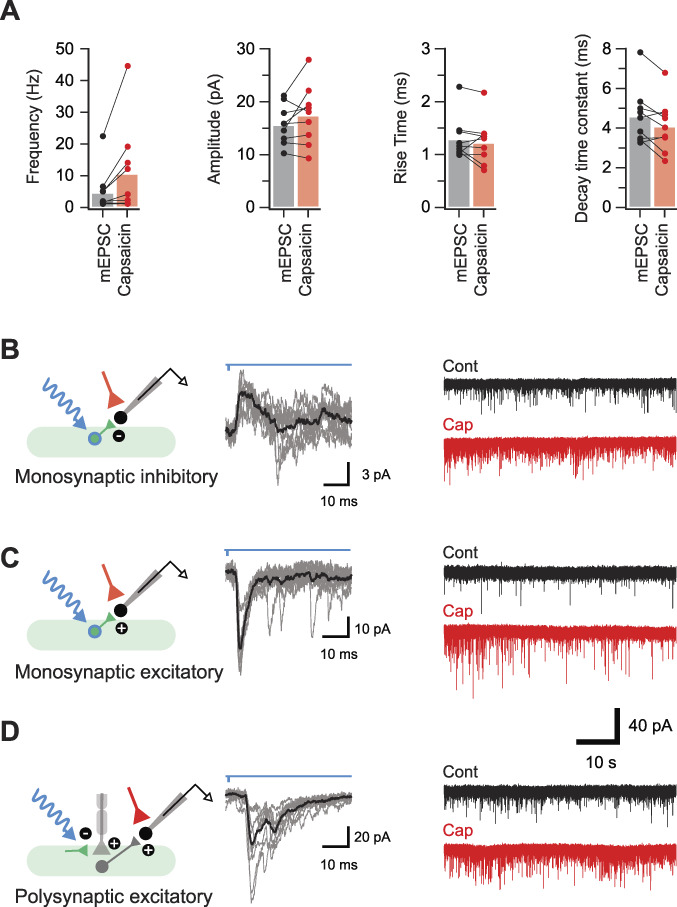
Parvalbumin-expressing interneurons modulate nociceptive circuits. (A) Plots show group data demonstrating the increase in mEPSC frequency after bath-applied capsaicin. Neurons were deemed to be capsaicin sensitive if capsaicin application increased mEPSC frequency by 3 standard deviations or more above the mean baseline rate. Miniature excitatory postsynaptic current amplitude and rise time remained unchanged, whereas decay time constant was reduced after capsaicin application. (B and C) Schematics (left) summarise the microcircuits producing photostimulation-evoked postsynaptic currents (oPSCs, 10 consecutive sweeps and average overlayed) in neurons from laminae I-IIo (middle). Continuous traces (right) show mEPSC recordings (TTX 1 μM, bicuculline 10 μM, and strychnine 1 μM) from corresponding neurons before (black) and after (red) bath application of capsaicin (2 μM). Capsaicin application increases mEPSC frequency without altering amplitude, confirming these neurons received nociceptive input. These recordings identified some neurons that received monosynaptic oIPSCs (B, short-latency outward currents during voltage clamp at −40 mV) presumably arising from photostimulation of iPVINs, neurons that received monosynaptic oEPSCs (C, short-latency inward currents during voltage clamp at −70 mV) arising from photostimulation of an ePVIN population, or neurons that received polysynaptic oEPSCs (D, longer-latency inward currents during voltage clamp at −70 mV) arising from photostimulation of iPVIN terminals that cause primary afferent depolarisation (PAD) and synaptic transmission at those terminals. ePVIN, excitatory parvalbumin-expressing interneuron; iPVIN, inhibitory parvalbumin-expressing interneuron; mEPSC, miniature excitatory postsynaptic current; oEPSC, optically evoked excitatory postsynaptic current; oIPSC, optically inhibitory postsynaptic current.
